
Predictors of primary cutaneous melanoma stage at diagnosis: observations from Alberta’s Tomorrow Project
Introduction
Melanoma mainly occurs in the skin (cutaneous melanoma) but also occurs in the eyes and intestines and is due to the uncontrolled growth of melanocytes (1). Melanoma has the highest mortality but lowest incidence of skin cancers in Canada compared to basal cell and squamous cell carcinoma (2). Incident rates of melanoma continue to increase in Canada. The average annual percent change (AAPC) in age-standardized incidence rates for males was 2.0 [95% confidence interval (CI): 1.8, 2.1] between 1984 and 2017 which was nearly identical for females (AAPC =2.0, 95% CI: 1.8, 2.2) between 1994 and 2017 (3). However, people diagnosed in stage I have a very favourable prognosis compared to those diagnosed at higher stages (4).
Risk factors associated with melanoma etiology are ultraviolet (UV) exposure (UVA and UVB), male sex, older age and the presence of melanocytic nevi (5). A recent systematic review found the association between UV exposure and the development of malignant melanoma is less evident in people with darker pigmented skin compared to individuals with lighter-pigmented skin (6). However, individuals with darker skin pigment are often diagnosed at a later stage leading to greater mortality despite the relatively lower incidence (6).
The stage of diagnosis of melanoma is a crucial predictor of survival and is determined primarily by tumor thickness. Other factors involved in stage determination include the presence of ulceration, nodal involvement and metastases that are associated with later stages (7-9). It is especially important to prevent late-stage diagnoses as the 5-year relative survival of stages III and IV melanoma is only 50% compared to 100% for those diagnosed at stage I (4).
Risk factors associated with later stage at diagnosis of melanoma include lower socioeconomic status (SES, measured as median income or using a Deprivation Index) (10), lower education, unemployment, smoking, being unmarried, history of sunburns and the anatomical location of the lesion such as the trunk or feet (10-14). Sunburns in childhood have been associated with increased risk of developing melanoma (15), while no association was found between increasing number of sunburns and later stage at diagnosis (16). Findings for anatomic locations associated with a later diagnosis are inconsistent in the literature (13-15,17). Other factors associated with later stage at diagnosis includes smoking (12), and male sex (11,12,15). Tumour thickness has been found to be associated with women (thinner tumours, ≤1 mm) (13) and people with obesity (increased Breslow tumor thickness) (11). Delay in seeking medical attention and diagnostic errors by clinicians are other notable factors (14).
Chronic disease and its management has emerged as a new epidemiological factor associated with later stage melanoma at diagnosis (18). A Spanish study found an association between pre-existing type 2 diabetes mellitus and melanoma stage at diagnosis regardless of diabetic pharmacotherapy management (18), while another study reported an association between use of anti-hypertensive drugs (diuretics and B-adrenergic blocking agents) and increased risk of developing melanoma (19). A Canadian study found increased exposure to thiazide diuretics linked to a higher risk of skin cancer including melanoma and keratinocyte carcinoma in seniors (20).
The epidemiology of cutaneous malignant melanoma in Canada is limited but a 2019 multi-center study that analyzed n=72,565 cutaneous melanoma cases found important trends including a slightly higher incidence rate in males versus females, and differences in most common anatomical location by sex (head and trunk most common in men and lower extremities in women). Important limitations of this comprehensive study were the lack of any data on disease stage at diagnosis including tumor thickness or lifestyle factors (21). Therefore, the objective of this current study was to address this gap in stage at diagnosis of melanoma by evaluating known and novel lifestyle and sociodemographic factors in the Canadian context.
Methods
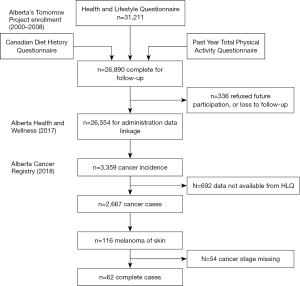
Alberta’s Tomorrow Project (ATP) is a prospective cohort that recruited adults with no history of cancer (except for non-melanoma skin cancer) aged 35–69 years living in Alberta, Canada from 2000 to 2015 (22,23). A total of 55,000 participants were enrolled and completed questionnaires at baseline and approximately every 4 years, including a health and lifestyle questionnaire (HLQ), Canadian Diet History Questionnaire-I (CDHQ-I) and the Past Year Total Physical Activity Questionnaire (PYTPAQ) (24-26). Participants also consented to regular linkage of questionnaire data to administrative health data, including the Alberta Cancer Registry (ACR) for identification of incident cancer cases. The participants diagnosed with an invasive melanoma were identified from phase I [2000–2008] of the ATP cohort as of January 2018. Figure 1 depicts how this study data set was obtained from the 3,359 cancer incident cases, including the 116 participants diagnosed with a cutaneous melanoma, leaving 62 participants for the current analysis. The missing stage information occurred during the time period when it was not routinely recorded in the ACR and was assumed to be missing at random.
Stage of melanoma at diagnosis was obtained through linkage with ACR and defined based the American Joint Committee on Cancer (AJCC) 7th edition staging guidelines. The AJCC 7th edition utilized the Tumor, Node and Metastases (TNM) staging system to classify melanomas based on pathological and morphological features (9,27). Stage I and II tumors typically lack lymph nodal involvement, stage III includes tumours of varying depths from 1 to >4 mm with lymph nodal involvement and stage IV disease involves distant metastases (8,9). Vertical depth of invasion remains one of the most important prognostic features but does not directly correlate to stage of disease (7).
Lifestyle factors evaluated in this study were based on a literature review of significant factors associated with melanoma stage, as well as others available in the ATP dataset. Factors measured by the HLQ included personal and family history of diseases, participation in organized cancer screening, smoking history, sun burns and sun exposure, body measurements including weight and height, and demographic information such as current employment, total household income before tax and education. The PYTPAQ queried household, occupational and recreational activities. Intensities of activity levels are reported as metabolic equivalents (METs), where one MET is defined as the amount of oxygen consumed while sitting at rest and is equal to 3.5 mL O2 per kilogram body weight per minute. A MET value of 1.3 is assigned to sitting in an office chair while standing is assigned a value of 3.0 or 4.5 depending on the nature of the work (28). The CDHQ-I is a past year food frequency questionnaire that assesses frequency of consumption and portion sizes of more than 100 common foods. Descriptions of the previously identified lifestyle variables that were available in the ATP data are provided in Table 1 and all of the variables considered in the analyses are given in the statistical analysis plan in Appendix 1.
Table 1
| Factors | Source | Question asked | Coding |
|---|---|---|---|
| Melanoma skin cancer stage | ACR administrative data linkage | – | I/II/III/IV |
| Age at baseline enrollment (years) | HLQ | – | Integer value |
| Age at cancer diagnosis (years) | ACR administrative data linkage | – | Integer value |
| Education | HLQ | 9 categories | Collapsed to high school/college/university degree |
| Married or common in-law | HLQ | 6 categories | Collapsed to married or common-law or not |
| Employment | HLQ | 7 categories | Collapsed to working full-time/part time, or not |
| Ethnicity | HLQ | 20 categories | Collapsed to white or not |
| Annual household income | HLQ | 11 categories | Collapsed to <$50,000/$50,000–100,000/>$100,000 |
| Geographic residence | Alberta Health administrative data linkage | Processed by the ATP | Urban/rural |
| Sunburn in the past year | HLQ | Q: In the past year, has any part of your body been sunburned? (a sunburn is any reddening or discomfort of your skin that lasts longer than 12 hours after exposure to the sun or other ultraviolet sources, such as tanning beds or sunlamps) | Yes/no |
| Untanned skin color of inner upper arm | HLQ | Q: Would you say that the untanned skin color of your inner upper arm is: | Collapsed to light/medium (no one with dark skin in this study) |
| Smoking status | HLQ | Multiple (>20) questions | Derived (by ATP) to current regular smoker/occasional smoker/past smoker/never smoker |
| Spend time in the sun 11 am–4 pm during the past June–August (hours) | HLQ | Q: During the past year June through August, on a typical day outdoors, approximately how much time did you spend in the sun between 11 am and 4 pm? (less than 30 minutes per day/30 minutes to <1 hour per day/1–2 hours per day/>2 hours per day) | Collapsed to <1, 1–2 and >2 hours |
| Total body mass index | HLQ | Calculated from the self-reported weight and height | Continuous |
| Total occupational physical activity (MET-hours/week) | Past Year Total Physical Activity Questionnaire | – | Continuous |
ACR, Alberta Cancer Registry; HLQ, Health and Lifestyle Questionnaire; ATP, Alberta’s Tomorrow Project; MET, metabolic equivalent.
ATP participants diagnosed at stages II, III & IV were combined in the current study due to small numbers (11 participants diagnosed in stage II and 9 participants in stages III and IV) as well as similar poorer survival outcomes (4). Missing values in lifestyle factors from the three questionnaires were minimal, but were imputed using mean (continuous variables) or reference category (binary or categorical variables). Descriptive analyses compared lifestyle and other factors between stage groups using Fisher’s exact tests or Mood’s test of medians. Logistic regression models evaluated individual factors previously identified in the literature, one at a time; those with a P<0.1 were then evaluated in a multivariable logistic regression model. Backward elimination removed factors with a P<0.05 to create a preliminary final model. A conditional variable importance measure from a Random Forest model (R package: partykit, v1.2-5) was used to identify possible other factors that were then forced into the preliminary final model and removed if their P value was greater than 0.05. The final model was evaluated for influential outliers and having a linear functional form (continuous variable). All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.2.1 (R Core Team).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval for this study (HREBA.CC—16-0814) and ATP (HREBA.CC—17-0461) was granted from the Health Research Ethics Board of Alberta—Cancer Committee. Informed consent was obtained from all participants involved in the study.
Results
Descriptive results are presented in Table 2. Half of the participants were female (50.0%), most lived in an urban setting (82.3%), were employed full-time or part-time (69.4%), had at least some post-secondary education (62.9%), were current or past smokers (58.1%) and were married or living common-law (83.9%). Most had light coloured skin measured in the inner part of the upper arm (80.6%), had a sunburn in the previous year (59.7%) and spent more than 1 hour in the sun between 11 am–4 pm per day during the past June through August (67.7%). The median age at diagnosis for males was 64 and for females 58 with nearly half of participants (48.4%) less than 60 years old at the time of diagnosis, family history of melanoma was low (8.1%) but higher for any type of cancer (56.5%). Factors that reached statistical significance (P<0.05) between the early and later stages at diagnosis included spending more than 1 hour in the sun 11 am–4 pm each day during the past June through August (P=0.04) and annual household income (P=0.01) with being married or common law close to this cutoff (P=0.06).
Table 2
| Factors | Stage I (n=42) | Stages II + III + IV (n=20) | Total (n=62) | P value (stage I vs. stages II + III + IV) |
|---|---|---|---|---|
| Gender | >0.99 | |||
| Male | 21 (50.0) | 10 (50.0) | 31 (50.0) | |
| Female | 21 (50.0) | 10 (50.0) | 31 (50.0) | |
| Married or common in-law | 0.06 | |||
| Yes | 38 (90.5) | 14 (70.0) | 52 (83.9) | |
| No | – | – | 10 (16.1) | |
| Age at baseline (years) | 0.14 | |||
| <60 | 33 (78.6) | 12 (60.0) | 45 (72.6) | |
| ≥60 | 9 (21.4) | 8 (40.0) | 17 (27.4) | |
| Age at diagnosis (years) | 0.79 | |||
| <60 | 21 (50.0) | 9 (45.0) | 30 (48.4) | |
| ≥60 | 21 (50.0) | 11 (55.0) | 32 (51.6) | |
| Education | 0.78 | |||
| ≤ High school | 15 (35.7) | 8 (40.0) | 23 (37.1) | |
| Some or completed post-secondary | 27 (64.3) | 12 (60.0) | 39 (62.9) | |
| Employment | 0.76 | |||
| Yes | 30 (71.4) | 13 (65.0) | 43 (69.4) | |
| No | 12 (28.6) | 7 (35.0) | 19 (30.6) | |
| Annual household income | 0.01* | |||
| <$50,000 | 8 (19.0) | 10 (50.0) | 18 (29.0) | |
| $50,000–$100,000 | 20 (47.6) | 9 (45.0) | 29 (46.8) | |
| >$100,000 | – | – | 15 (24.2) | |
| Geographic residence | 0.73 | |||
| Urban | 35 (83.3) | 16 (80.0) | 51 (82.3) | |
| Rural | – | – | 11 (17.7) | |
| Sunburn in the past year | 0.10 | |||
| Yes | 22 (52.4) | 15 (75.0) | 37 (59.7) | |
| No or missing | – | – | 25 (40.3) | |
| Untanned skin color of inner upper arm | 0.30 | |||
| Light | 32 (76.2) | 18 (90.0) | 50 (80.6) | |
| Medium (no dark) | – | – | 12 (19.4) | |
| Number of hours spent in the sun 11 am–4 pm per day during the past June through August | 0.04* | |||
| <1 hour | 10 (23.8) | 10 (50.0) | 20 (32.3) | |
| 1–2 hours | 15 (35.7) | 2 (10.0) | 17 (27.4) | |
| >2 hours | 17 (40.5) | 8 (40.0) | 25 (40.3) | |
| Smoking status | 0.78 | |||
| Current or past smoker | 25 (59.5) | 11 (55.0) | 36 (58.1) | |
| Never smoked | 17 (40.5) | 9 (45.0) | 26 (41.9) | |
| Age at enrollment (years) | 52.0 (46.0, 58.8) | 57.5 (44.3, 63.3) | 52.5 (45.3, 62.0) | 0.09 |
| Age at diagnosis (years) | 59.5 (52.3, 68.8) | 63.0 (51.0, 69.0) | 60.0 (51.3, 68.8) | 0.54 |
| Body mass index (kg/m2) | 27.3 (24.9, 30.3) | 29.9 (25.0, 35.0) | 27.7 (24.9, 31.2) | 0.31 |
| Occupational METs | 68.0 (16.7, 90.2) | 82.4 (44.6, 138.7) | 74.6 (32.0, 96.4) | 0.29 |
Data are presented as n (%) or median (Q1, Q3). One MET =3.5 mL O2 per kilogram body weight per minute. *, statistical significance at P<0.05 based on Fisher’s exact test or Mood’s test of medians. “–” indicates cell counts suppressed. Q1, 25th percentile; Q3, 75th percentile; METs, metabolic equivalents.
The univariate logistic regression model identified 11 factors while the Random Forest identified ten of these factors. The small number of participants with a family history of melanoma meant it was neither a useful nor statistically significant predictor variable (P=0.97) and was not forced into the final model. Following backward elimination, three variables remained. In the final multivariable logistic regression model for stage I versus higher stages at diagnosis, a protective effect for individuals with the highest annual household income (>$100,000) compared to the lowest tier [<$50,000; odds ratio (OR) =0.069, 95% CI: 0.006, 0.733, P=0.02] was found (Table 3). There was also a protective effect associated with longer outdoor summer exposure during the hours 11 am to 4 pm, with an OR =0.153 (95% CI: 0.024, 0.976, P=0.04) for 1–2 versus <1 hour. Increasing occupational MET-hours, defined as MET-hours acquired in an occupational activity in hours/week, was found to be associated with an increased risk of later stage at melanoma diagnosis (OR =1.017, 95% CI: 1.003, 1.031, P=0.02).
Table 3
| Factors | OR (95% Wald CI) | P value |
|---|---|---|
| Annual household income | ||
| >$100,000 versus <$50,000 | 0.069 (0.006–0.733) | 0.02 |
| $50,000–$100,000 versus <$50,000 | 0.360 (0.090–1.448) | 0.15 |
| Number of hours spent in the sun 11 am–4 pm per day during the past June through August | ||
| 1–2 versus <1 hour | 0.153 (0.024–0.976) | 0.04 |
| >2 versus <1 hour | 0.235 (0.048–1.156) | 0.07 |
| Past year occupational activity (MET-hours/week) | 1.017 (1.003–1.031) | 0.02 |
One MET =3.5 mL O2 per kilogram body weight per minute. OR, odds ratio; CI, confidence interval; MET, metabolic equivalent.
Discussion
The protective effect of higher SES associated with stage I at diagnosis is consistent with previously published literature and thought to be associated with greater access to health care. The protective effect of longer outdoor time spent in the sun during the summer months could be due to multiple factors: people could be more likely to use sun protection than during other times of the day or check their skin more often leading to early detection (29) or being more physically active, which is a healthy lifestyle surrogate (30). However, no association between increasing time for outdoor recreational activity and tumor depth was found by a German retrospective cohort of n=233 primary cutaneous melanoma cases (16). Thus, the association between outdoor recreational activity and stage of diagnosis of melanoma remains unclear.
Increasing occupational MET-hours was associated with a later stage at diagnosis. A Western Canada case-control study of n=595 melanoma cases found an association between increasing occupational physical activity and risk of developing cutaneous melanoma but only among those with the highest levels of occupational physical activity (31). This was a weak statistically significant association without a clear dose-response relationship, even when adjusting for sunlight exposure at work (31). A challenge when evaluating occupational physical activity and risk of developing or stage at diagnosis of melanoma is the lack of corresponding information on UV radiation exposure, other physical activity domains and other occupational exposures (32). The findings of the case-control study and the current study highlights the need for further research that will discern the role of occupational physical activity and melanoma.
Our study did not yield an association between stage of melanoma diagnosis and physical activity (outside of occupational MET-hours) which is consistent with other studies. A Norwegian study of 1,444 women aged 30–75 years with primary invasive melanoma also did not identify a link between the two variables (33). Our study also did not find an association between sunburns and stage at melanoma diagnosis, perhaps due to the relatively small sample size and lack of information on burn severity and body surface distribution. Lastly, the lack of association observed with chronic disease in this cohort could be due to the fact that most participants were relatively healthy at recruitment and did not have chronic disease at the time of completing the questionnaires.
A major limitation of this study is the small number of participants that would limit statistical power to identify associations. In addition, most of the participants had light coloured skin, and only 12 had medium coloured skin, limiting the ability to analyze by skin colour or detect an association with disease stage. We also did not have data on the ulcerative and mitotic information or anatomical site of the melanoma, although no known association with anatomical site and later stage at diagnosis has been found. Lastly, information on tanning bed use was also not available which contributes to melanoma risk but its association with stage at diagnosis is unknown.
This is one of the first Canadian prospective cohort studies to examine the epidemiologic factors associated with stage at diagnosis of melanoma—the 7th and 8th most common cancers in Canadian females and males, respectively (3). Individuals with lighter-pigmented skin continue to experience the greatest increases in incidence, highlighting the continued relevance of this study population which was predominantly Caucasian (34). This study directly builds on the two most recent Canadian epidemiological studies of melanoma. The study by Ghazawi et al. illustrated important trends in the incidence of the disease across the country, including geographical and sex-specific mortality outcomes (21). However, it did not have any data on disease stage or tumor depth at diagnosis, which is a hallmark of this study. The second study by this group shared these same data limitations but did identify geographic regions in Canada based on postal codes with higher incidence rates, including two locations in Alberta (35). Another important strength of this study is that it investigated the association between lifestyle factors known to be associated with melanoma such as smoking and rural versus urban living, and novel factors that have emerged recently such as chronic disease. All data collection for ATP was completed prior to the COVID-19 pandemic and therefore there are no issues with the tracking of the lifestyle factors during the data recruitment phase of the study
Conclusions
This study provided a comprehensive snapshot of the epidemiology of melanoma using high-quality data from a large cohort. Identifying factors associated with early stage at diagnosis is especially important for melanoma, given the significant decrease in survival outcomes for those diagnosed in stage II or higher and the increasing incidence rates. Lifestyle factors are especially important to identify given that many of them are modifiable and allow for cancer prevention. Future research that can incorporate lifestyle factors, as well as sun exposure behaviours over the life course will be able to further understand their role in diagnosing melanoma at an early stage.
Acknowledgments
Alberta’s Tomorrow Project is only possible because of the commitment of its research participants, staff, and funders: Alberta Health, Alberta Cancer Foundation, Canadian Partnership Against Cancer, and Health Canada, and substantial in-kind funding from Alberta Health Services. ACR data were obtained through linkage with Surveillance and Reporting, Cancer Research and Analytics, and Cancer Care Alberta. The views expressed herein represent the views of the author(s) and not those of Alberta’s Tomorrow Project or any of its funders. This study was based in part on data provided by Alberta Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta. Neither the Government nor Alberta Health expressed any opinion in relation to this study. Access to individual-level data is available in accordance with the Health Information Act of Alberta and Alberta’s Tomorrow Project (ATP) Access Guidelines at https://myatpresearch.ca/DataAccess (accessed on 11 January 2018).
Funding: This work was supported by
Footnote
Peer Review File: Available at https://ace.amegroups.com/article/view/10.21037/ace-23-7/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ace.amegroups.com/article/view/10.21037/ace-23-7/coif). P.J.R. is the Chair of the Board of the Canadian Cancer Research Alliance. This is a volunteer position, and she doesn’t receive stipend. However, P.J.R. is reimbursed for travel and accommodation for two meetings per year. Reimbursement is at the government rate for economy travel. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval for this study (HREBA.CC—16-0814) and ATP (HREBA.CC—17-0461) was granted from the Health Research Ethics Board of Alberta—Cancer Committee. Informed consent was obtained from all participants involved in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cancer Institute. What Does Melanoma Look Like? National Institutes of Health; 2011. Available online: https://www.cancer.gov/types/skin/melanoma-photos
- Public Health Agency of Canada. Melanoma Skin Cancer: Government of Canada; 2019. Available online: https://www.canada.ca/en/public-health/services/chronic-diseases/cancer/melanoma-skin-cancer.html
- Brenner D, Poirier A, Smith L. Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Society; 2021.
- Cancer Care Alberta. The 2021 Report on Cancer Statistics in Alberta: Surveillance & Reporting, Cancer Research & Analytics, Cancer Care Alberta, Alberta Health Services; 2021. [updated December 4, 2022]. Available online: https://public.tableau.com/app/profile/cancercontrol.ab/viz/The2021ReportonCancerStatisticsinAlberta/Highlights
- Dzwierzynski WW. Melanoma Risk Factors and Prevention. Clin Plast Surg 2021;48:543-50. [Crossref] [PubMed]
- Lopes FCPS, Sleiman MG, Sebastian K, et al. UV Exposure and the Risk of Cutaneous Melanoma in Skin of Color: A Systematic Review. JAMA Dermatol 2021;157:213-9. [Crossref] [PubMed]
- Melanoma Research Alliance. Understanding Melanoma Staging. Melanoma Research Alliance. Available online: https://www.curemelanoma.org/about-melanoma/melanoma-staging/understanding-melanoma-staging
- Chu VH, Tetzlaff MT, Torres-Cabala CA, et al. Impact of the 2009 (7th edition) AJCC melanoma staging system in the classification of thin cutaneous melanomas. Biomed Res Int 2013;2013:898719.
- American Cancer Society. Melanoma Skin Cancer Early Detection, Diagnosis, and Staging. American Cancer Society; 2023. Available online: https://www.cancer.org/cancer/types/melanoma-skin-cancer/detection-diagnosis-staging.html
- Jiang AJ, Rambhatla PV, Eide MJ. Socioeconomic and lifestyle factors and melanoma: a systematic review. Br J Dermatol 2015;172:885-915. [Crossref] [PubMed]
- Skowron F, Bérard F, Balme B, et al. Role of obesity on the thickness of primary cutaneous melanoma. J Eur Acad Dermatol Venereol 2015;29:262-9. [Crossref] [PubMed]
- Van Durme DJ, Ferrante JM, Pal N, et al. Demographic predictors of melanoma stage at diagnosis. Arch Fam Med 2000;9:606-11. [Crossref] [PubMed]
- Talaganis JA, Biello K, Plaka M, et al. Demographic, behavioural and physician-related determinants of early melanoma detection in a low-incidence population. Br J Dermatol 2014;171:832-8. [Crossref] [PubMed]
- Albreski D, Sloan SB. Melanoma of the feet: misdiagnosed and misunderstood. Clin Dermatol 2009;27:556-63. [Crossref] [PubMed]
- Gajda M, Kaminska-Winciorek G. Do not let to be late: overview of reasons for melanoma delayed diagnosis. Asian Pac J Cancer Prev 2014;15:3873-7. [Crossref] [PubMed]
- Schmid-Wendtner MH, Baumert J, Stange J, et al. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res 2002;12:389-94. [Crossref] [PubMed]
- O'Shea SJ, Rogers Z, Warburton F, et al. Which symptoms are linked to a delayed presentation among melanoma patients? A retrospective study. BMC Cancer 2017;17:5. [Crossref] [PubMed]
- Nagore E, Martinez-Garcia MA, Gomez-Olivas JD, et al. Relationship between type 2 diabetes mellitus and markers of cutaneous melanoma aggressiveness: an observational multicentric study in 443 patients with melanoma. Br J Dermatol 2021;185:756-63. [Crossref] [PubMed]
- Tang H, Fu S, Zhai S, et al. Use of Antihypertensive Drugs and Risk of Malignant Melanoma: A Meta-analysis of Observational Studies. Drug Saf 2018;41:161-9. [Crossref] [PubMed]
- Drucker AM, Hollestein L, Na Y, et al. Association between antihypertensive medications and risk of skin cancer in people older than 65 years: a population-based study. CMAJ 2021;193:E508-16. [Crossref] [PubMed]
- Ghazawi FM, Cyr J, Darwich R, et al. Cutaneous malignant melanoma incidence and mortality trends in Canada: A comprehensive population-based study. J Am Acad Dermatol 2019;80:448-59. [Crossref] [PubMed]
- Robson PJ, Solbak NM, Haig TR, et al. Design, methods and demographics from phase I of Alberta's Tomorrow Project cohort: a prospective cohort profile. CMAJ Open 2016;4:E515-27. [Crossref] [PubMed]
- Ye M, Robson PJ, Eurich DT, et al. Cohort Profile: Alberta's Tomorrow Project. Int J Epidemiol 2017;46:1097-1098l. [Crossref] [PubMed]
- Alberta’s Tomorrow Project. Health and Lifestyle Questionnaire (HLQ) Data Dictionary. Available online: https://myatpresearch.ca/data-dictionary/hlq/
- Csizmadi I, Kahle L, Ullman R, et al. Adaptation and evaluation of the National Cancer Institute's Diet History Questionnaire and nutrient database for Canadian populations. Public Health Nutr 2007;10:88-96. [Crossref] [PubMed]
- Friedenreich CM, Courneya KS, Neilson HK, et al. Reliability and validity of the Past Year Total Physical Activity Questionnaire. Am J Epidemiol 2006;163:959-70. [Crossref] [PubMed]
- Melanoma Skin Cancer Stages. American Cancer Society; 2019.
- Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575-81. [Crossref] [PubMed]
- Jongenelis M, Pettigrew S, Strickland M, et al. The relationship between skin checking and sun protection behaviours: implications for skin cancer prevention campaigns. Public Health 2018;155:55-8. [Crossref] [PubMed]
- Beyer KMM, Szabo A, Hoormann K, et al. Time spent outdoors, activity levels, and chronic disease among American adults. J Behav Med 2018;41:494-503. [Crossref] [PubMed]
- Lee TK, MacArthur AC, Gallagher RP, et al. Occupational physical activity and risk of malignant melanoma: the Western Canada Melanoma Study. Melanoma Res 2009;19:260-6. [Crossref] [PubMed]
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR 2017;24:8-14. [PubMed]
- Perrier F, Robsahm TE, Ghiasvand R, et al. No association between physical activity and primary melanoma thickness in a cohort of Norwegian women. Br J Dermatol 2023;188:670-90. [Crossref] [PubMed]
- Arnold M, Singh D, Laversanne M, et al. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol 2022;158:495-503. [Crossref] [PubMed]
- Conte S, Ghazawi FM, Le M, et al. Population-Based Study Detailing Cutaneous Melanoma Incidence and Mortality Trends in Canada. Front Med (Lausanne) 2022;9:830254. [Crossref] [PubMed]
Cite this article as: Ghebrial M, Wang Q, Zhang R, Robson PJ, Shack L, Kopciuk KA. Predictors of primary cutaneous melanoma stage at diagnosis: observations from Alberta’s Tomorrow Project. Ann Cancer Epidemiol 2024;8:1.


