
Cervical cancer in Saudi Arabia: trends in survival by stage at diagnosis and geographic region
Introduction
The global range of age-standardised five-year net survival for women diagnosed with cervical cancer during 2010–2014 is wide, from less than 40% in some African countries to over 70% in Northern Europe (1).
Five-year survival for women diagnosed in Saudi Arabia between 1995 and 2004 has been reported (2). More detailed analysis by stage at diagnosis is important for interpretation of survival data, which is crucial to inform healthcare policy. Further, no research has yet been done to identify any regional disparities in cancer outcomes in Saudi Arabia. Healthcare in Saudi Arabia is provided free of charge by different government sectors, but the concentration of oncologists and modern treatment facilities in major cities may pose a challenge to equitable access to treatment (3,4). Regional variation and time trends in survival would enable assessment of the effectiveness of the healthcare system in treatment and control, reflecting the availability of early diagnosis, thorough investigation and effective treatment (5). Examining trends in survival by stage at diagnosis will help to determine both the effectiveness of diagnostic activity and the availability and efficacy of stage-appropriate treatment.
Although relatively uncommon in Saudi Arabia and the Middle East in general, the availability of affordable and safe methods for prevention and early detection means that the majority of cervical cancer deaths are avoidable with relatively simple public health interventions (6). Therefore, in November 2020, the World Health Organisation launched a global strategy to eliminate cervical cancer as a public health problem (7). The strategy includes interim aims to screen 70% of women twice (by age 35 and again by age 45) using a high-performance test, and to treat 90% of women with identified cervical pre-cancer and 90% of women with invasive cervical cancer.
We provide up-to-date and detailed survival estimates for women diagnosed with cervical cancer in Saudi Arabia between 2005 and 2016. We explore survival trends by stage at diagnosis and administrative region. We examine whether any regional discrepancies in survival are due to differences in the distribution of stage at diagnosis or in stage-specific survival. We present the following article in accordance with the STROBE reporting checklist (available at https://ace.amegroups.com/article/view/10.21037/ace-22-2/rc).
Methods
The study was conducted as a retrospective cohort in which cancer registry and government records were utilised to obtain data on cervical cancer diagnosis and vital status at five years after diagnosis, respectively.
Ethical approval was obtained from the London School of Hygiene and Tropical Medicine (#14739, 17 January 2018) and individual consent for this retrospective analysis was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data sources
We obtained data on all 2,330 women diagnosed with cervical cancer in Saudi Arabia between January 2005 and December 2016 from the national population-based Saudi Cancer Registry (SCR). The data included full dates of birth, diagnosis and last known vital status, the administrative region of residence and of diagnosis; as well as nationality, tumour stage [Surveillance, Epidemiology and End Results (SEER) Summary Stage 2000], grade, morphology, behaviour, the date and cause of death if dead, as stated on a death notification or death certificate if received by the registry (cancer, other or unknown). Detailed data on stage at diagnosis are collected from pathology reports. SEER summary stage is then determined by trained registrars as localised [confined to uterine cervix, International Federation of Gynecology and Obstetrics (FIGO) stage I]; regional by direct extension to adjacent organs or structures, regional lymph node involvement, or both (FIGO stage II or III), and distant organ or lymph node metastasis (FIGO stage IV) (8). An assessment of data quality, including the validity of stage data, was made internally in 2014 by re-abstraction of data in a random sample of medical records from each region. The data were found to be highly concordant. Further details of cancer registration in Saudi Arabia have been published (9).
Non-Saudi nationals often live in Saudi Arabia on work-related visas of limited duration. Many return to their home countries after a diagnosis of cancer, making follow-up difficult. Obtaining accurate life tables for this population is challenging, given their unsteady in- and out-migration. Therefore, we limited the survival analyses to Saudi women.
Of 1,477 records for Saudi women with an invasive primary cervical neoplasm, 1,199 (81%) had at least one complete (10-digit) national ID number, while 31 women had two ID numbers. Tumour registrars occasionally find more than one ID number in a woman’s medical record, one of which probably belongs to an accompanying husband or relative. All available ID numbers were submitted to the National Information Centre (NIC) of the Ministry of Interior. The vital status and if dead, the date of death, were requested, as well as sex, date of birth and complete name (first name, father, grandfather, and family name) to enable comparison with registry records. Linkage was successful for 1,193 ID numbers on 29 August 2019, corresponding to 1,191 registry records. Eighty-three of the ID numbers in the NIC belonged to males. This eliminated 29 of the second ID numbers in the records of women with two IDs. For the remaining two records, the correct ID number was selected based on matching names and dates of birth. The remaining 54 IDs belonging to males were therefore considered to be wrong ID numbers (Figure 1). For the 373 women with missing, incomplete or wrong ID numbers, the date of last known vital status in the registry was used in the survival analyses. For 20 women, linkage of the ID in the tumour registration with NIC records did not reveal a date of death, even though the registry record showed them as having died. We treated these women as alive on 29 August 2019 in the survival analyses, for consistency.
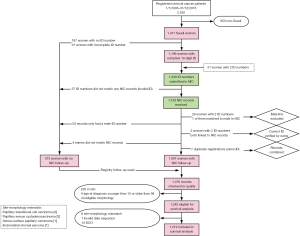
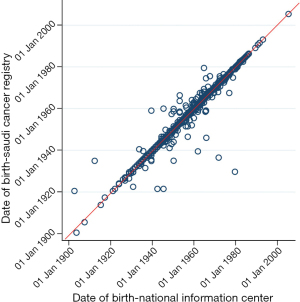
Six further records had names that did not completely match between registry and NIC records. Registry follow-up was used if more than one of the four names did not match (n=4). There was a much higher proportion of mismatch in dates of birth between registry and NIC records, ranging between 1 day and 24 years (Figure 2). Only 44% had perfectly matching dates of birth, while 23% had differences within 2 days, 19% within 1 year and 11% within 5 years. Many of the elderly in Saudi Arabia do not know their exact date of birth and are therefore assigned the mid-year date. In addition, the conversion from the locally used Hijri lunar calendar to the Gregorian calendar can lead to differences of a few days. Dates of birth from the registry were used for all analyses.
A total of 1,470 records were checked for CONCORD eligibility criteria (1). Patients were eligible for survival analysis if they had a complete date of birth, diagnosis and death if dead, were 15–99 years of age at diagnosis and if they had an invasive primary tumour with an eligible morphology and topography code. Further, patients were excluded from the analysis if registered based on death certificate only, or had inconsistent age, sex, morphology or site combinations (Figure 1, Table S1). Of the 1,242 women eligible for survival analysis, 1,219 (98.1%) were included in the survival analyses.
To control for background mortality in the estimation of net survival, we obtained life tables of all-cause mortality rates for women in Saudi Arabia, by single calendar year and 5-year age group, from the United Nations Population Division (UNPD). We interpolated them and extended them to age 99 years with the Elandt-Johnson method to obtain mortality rates by single year of age (10).
Statistical analysis
We estimated five-year net survival probabilities and their 95% confidence intervals (CIs) for women diagnosed during the two calendar periods 2005–2010 and 2011–2016, both for the whole population, and for each of the three main administrative regions (Riyadh, Makkah and the Eastern Region) and the other ten regions combined. This was to ensure that enough women were available for robust estimation of survival in each category, given the small population of the other ten regions.
Probabilities were estimated with the Pohar-Perme estimator (11) in Stata IC 16 version 1, using the program stns (12). Death due to any cause was the event of interest. Life tables of all-cause mortality rates in the Saudi Arabian female population were used to correct for background mortality. Women who were alive on 31 December 2018 were censored. For the 291 (24%) women for whom ID number was not available, follow-up time was censored at the date when they were last known to be alive to the registry. The date of death in the registry was used for women known to be dead.
A cohort approach was followed for women diagnosed during 2005–2010, for whom at least 5 years of potential follow-up were available. For the period 2011–2016, a complete approach was used, where women diagnosed in calendar years for which 5 years of follow-up were not available [2014–2016] were censored at the closing date (13).
We estimated one- and five-year net survival for each calendar period, and by region of residence and stage at diagnosis. Where at least 10 women were available for analysis in each age group, we produced estimates for each of five age groups (15–44, 45–54, 55–64, 65–74, and 75–99 years). An age-standardised summary estimate was derived using the International Cancer Survival Standard (ICSS) group 2 weights (cancers for which incidence is fairly constant with age) (14). If fewer than 10 women were at risk for a single age group, they were combined with the women in the adjacent age group and the resulting survival estimate was assigned to both age groups, which were then used for age standardisation. If fewer than 10 women were at risk for two or more age groups, we only present the unstandardised estimate for all ages combined. Survival estimates were not age-standardised if fewer than 50 women were at risk in an analysis stratum. Further, estimates were not age-standardised if, for at least one of the age groups with 10 or more women, the last event occurred before 6 months for 1-year estimates or before 3 years for 5-year estimates, and some women are still alive at the end of follow-up. This was done in order to obtain robust estimates, based on past experience from the CONCORD programme (1).
For 11.3% of women for whom stage was missing, we imputed stage using a multinomial logistic model that included the Nelson-Aalen estimate of the cumulative hazard, the event indicator (death), and dummy variables for grade, age group and region of diagnosis. We generated five imputed datasets. The resulting complete datasets had very similar stage distributions (Table 1).
Table 1
| Imputation | Localised, n (%) | Regional, n (%) | Distant, n (%) | Total |
|---|---|---|---|---|
| Complete observations | 378 (34.97) | 480 (44.40) | 223 (20.63) | 1,081 |
| 1 | 437 (35.88) | 534 (43.84) | 247 (20.28) | 1,218 |
| 2 | 433 (35.55) | 536 (44.01) | 249 (20.44) | 1,218 |
| 3 | 442 (36.29) | 528 (43.35) | 248 (20.36) | 1,218 |
| 4 | 448 (36.78) | 519 (42.61) | 251 (20.61) | 1,218 |
| 5 | 441 (36.21) | 533 (43.76) | 244 (20.03) | 1,218 |
We estimated age-standardised net survival for each of the complete datasets by stage and period of diagnosis. We then combined the point estimates of survival and their variance, using Rubin’s rule, to obtain a single pooled point estimate. We further calculated the between-imputation variance to account for the extra uncertainty from the missing data (15). We combined the two variances to obtain the variance for the pooled estimate and to derive its 95% CIs.
We estimated net survival by region of residence and stage at diagnosis without age standardisation for the 12-year period 2005–2016 to avoid small numbers in older age groups. The comparability of estimates between regions is not expected to be compromised since the age distribution of women with cervical cancer was similar between regions.
Results
Study population
The mean age at diagnosis with invasive cervical cancer was 53 years. The proportion of women diagnosed at a localised stage increased slightly from 2005–2010 to 2011–2016, but the proportion diagnosed at a regional stage decreased. There was minimal change in distant stage. Virtually all diagnoses were pathologically confirmed. The proportion of women with NIC follow-up increased between 2005–2010 and 2011–2016 but the proportion with a censored survival time was higher in 2011–2016 because women diagnosed in 2014–2016 did not have five full years of follow-up (Table 2).
Table 2
| Characteristic | 2005–2010, n (%) | 2011–2016, n (%) | All periods, n (%) |
|---|---|---|---|
| Age (years), mean ± SD | 52.70±13.59 | 53.73±13.47 | 52.8±13.52 |
| Region of residence | |||
| Riyadh | 145 (26.90) | 174 (25.59) | 319 (26.17) |
| Makkah | 157 (29.13) | 204 (30.00) | 361 (29.61) |
| Eastern | 92 (17.07) | 122 (17.94) | 214 (17.56) |
| Other | 142 (26.35) | 177 (26.03) | 319 (26.17) |
| Unknown | 3 (0.56) | 3 (0.44) | 6 (0.49) |
| Region of diagnosis | |||
| Riyadh | 249 (46.20) | 306 (45.00) | 555 (45.53) |
| Makkah | 171 (31.73) | 218 (32.06) | 389 (31.91) |
| Eastern | 55 (10.20) | 87 (12.97) | 142 (11.65) |
| Other | 63 (11.69) | 68 (10.00) | 131 (10.75) |
| Unknown | 1 (0.19) | 1 (0.15) | 2 (0.16) |
| Stage | |||
| Localised | 153 (28.39) | 225 (33.09) | 378 (31.01) |
| Regional | 234 (43.41) | 246 (36.18) | 480 (39.38) |
| Distant | 96 (17.81) | 127 (18.68) | 223 (18.29) |
| Unknown | 56 (10.39) | 82 (12.06) | 138 (11.32) |
| Grade | |||
| I | 38 (7.05) | 60 (8.82) | 98 (8.04) |
| II | 191 (35.44) | 205 (30.15) | 396 (32.49) |
| III | 136 (25.23) | 207 (30.44) | 343 (28.00) |
| IV | 23 (4.27) | 18 (2.65) | 41 (3.36) |
| Unknown | 151 (28.01) | 190 (27.94) | 341 (27.97) |
| Basis of diagnosis | |||
| Pathology | 533 (98.89) | 674 (99.12) | 1,207 (99.02) |
| Clinical/imaging | 2 (0.37) | 2 (0.29) | 4 (0.33) |
| Unknown | 4 (0.74) | 4 (0.59) | 8 (0.66) |
| NIC follow-up available | 377 (69.90) | 551 (81.00) | 928 (76.10) |
| Censored within 5 years of diagnosis | 130 (24.10) | 284 (41.70) | 414 (34.00) |
| Total | 539 | 680 | 1,219 |
SD, standard deviation; NIC, National Information Center.
Age-specific net survival
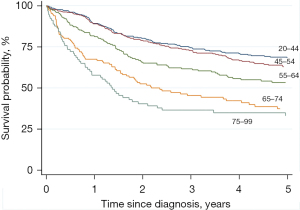
Net survival decreased with increasing age at diagnosis especially for those aged 65 and older (Table 3, Figure 3).
Table 3
| Age group (years) | n | 1-year | 5-year | |||
|---|---|---|---|---|---|---|
| Net survival | 95% CI | Net survival | 95% CI | |||
| 15–44 | 364 | 89.2 | 85.8–92.6 | 68.7 | 62.9–73.7 | |
| 45–54 | 362 | 88.9 | 85.5–92.3 | 63.6 | 57.2–68.6 | |
| 55–64 | 237 | 81.2 | 75.8–86.5 | 52.8 | 45.9–60.7 | |
| 65–74 | 155 | 67.5 | 59.7–75.3 | 39.1 | 28.5–46.3 | |
| 75+ | 101 | 57.8 | 47.3–68.2 | 30.1 | 22.7–43.5 | |
CI, confidence interval; ICSS, International Cancer Survival Standard.
Time trends in age-standardised net survival
From 2005–2010 to 2011–2016, age-standardised net survival for women from all regions and all stages combined did not change.
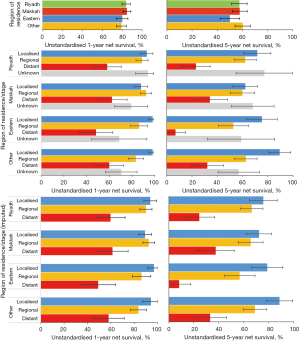
The regional pattern of survival did change. Women living in the Makkah region moved from having the lowest 5-year net survival during 2005–2010 (49.9%; 95% CI: 39.4–60.3%) to the highest during 2011–2016 (69.1%; 60.1–78.0%), while survival for women living in Riyadh, the Eastern Region and the other 10 regions combined remained the same or fell slightly. There was a small increase in 5-year net survival for women who were diagnosed at a localised or regional stage, and a decline for those diagnosed at a distant stage, or with unknown stage. Survival for women with unknown stage was similar to that for women diagnosed at a localised or regional stage. Stage-specific net survival did not change after imputing stage where it was missing (Table 4, Figure 4).
Table 4
| Period of diagnosis | n | 1-year | 5-year | ||||
|---|---|---|---|---|---|---|---|
| Net survival | 95% CI | Net survival | 95% CI | ||||
| Calendar period | 2005–10 | 539 | 81.0 | 76.8–85.2 | 59.2 | 52.7–65.7 | |
| 2011–16 | 680 | 79.4 | 75.8–83.0 | 59.7 | 54.7–64.6 | ||
| Region of residence | |||||||
| Riyadh | 2005–10 | 145 | 84.5 | 77.4–91.6 | 59.0 | 48.6–69.3 | |
| 2011–16 | 174 | 77.1 | 70.1–84.2 | 55.8 | 47.3–64.2 | ||
| Makkah | 2005–10 | 157 | 80.5 | 72.9–88.1 | 49.9 | 39.4–60.3 | |
| 2011–16 | 204 | 84.6 | 78.7–90.4 | 69.1 | 60.1–78.0 | ||
| Eastern | 2005–10 | 92 | 79.6 | 70.7–88.6 | 52.9 | 42.4–63.4 | |
| 2011–16 | 122 | 79.1 | 71.0–87.3 | 53.4 | 42.8–64.0 | ||
| Other | 2005–10 | 142 | 78.2 | 71.3–85.1 | 64.8 | 53.8–75.9 | |
| 2011–16 | 177 | 74.1 | 66.8–81.3 | 57.6 | 48.8–66.4 | ||
| Stage | |||||||
| Localised | 2005–10 | 153 | 88.1 | 80.3–95.8 | 67.4 | 57.3–77.5 | |
| 2011–16 | 225 | 90.0 | 84.1–95.9 | 73.8 | 63.5–84.1 | ||
| Regional | 2005–10 | 234 | 86.4 | 81.0–91.8 | 59.4 | 49.7–69.0 | |
| 2011–16 | 246 | 83.5 | 78.0–89.0 | 66.5 | 58.8–74.3 | ||
| Distant | 2005–10 | 96 | 61.9 | 52.1–71.8 | 32.1 | 22.2–42.0 | |
| 2011–16 | 127 | 55.6 | 46.5–64.7 | 25.2 | 16.6–33.8 | ||
| Unknown | 2005–10* | 56 | 84.8 | 73.1–96.4 | 72.0 | 56.7–87.4 | |
| 2011–16 | 82 | 70.4 | 58.6–82.2 | 63.4 | 49.9–77.0 | ||
| Stage after imputation§ | |||||||
| Localised | 2005–10 | 879 | 88.2 | 80.7–95.8 | 69.6 | 59.3–79.9 | |
| 2011–16 | 1304 | 89.9 | 84.2–95.7 | 74.3 | 64.9–83.6 | ||
| Regional | 2005–10 | 1282 | 85.8 | 80.5–91.1 | 60.8 | 51.3–70.3 | |
| 2011–16 | 1376 | 84.3 | 78.9–89.6 | 68.2 | 60.5–75.9 | ||
| Distant | 2005–10 | 529 | 61.7 | 52.2–71.3 | 32.0 | 22.0–41.9 | |
| 2011–16 | 720 | 54.3 | 45.1–63.6 | 24.9 | 16.3–33.4 | ||
*, unstandardised; §, n is the total from five imputations. CI, confidence interval.
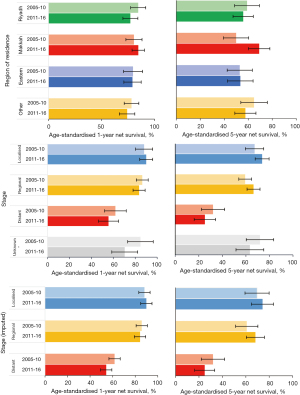
Unstandardised stage-specific net survival for women diagnosed during the 12-year period 2005–2016 was similar between regions except for women diagnosed at a distant stage, for which 5-year net survival in the Eastern region was remarkably lower than that in all other regions (Table 5, Figure 5). When including unknown stage as a category using the unimputed dataset, there was no regional difference in net survival for women with unknown stage.
Table 5
| Region | N | 1-year | 5-year | ||||
|---|---|---|---|---|---|---|---|
| Net survival | 95% CI | Net survival | 95% CI | ||||
| Region | Riyadh | 319 | 84.0 | 79.8–88.2 | 57.6 | 51.5–63.7 | |
| Makkah | 361 | 84.8 | 80.9–88.7 | 57.8 | 51.9–63.8 | ||
| Eastern | 214 | 80.1 | 74.5–85.8 | 50.0 | 42.4–57.7 | ||
| Other | 319 | 79.3 | 74.4–84.1 | 60.2 | 54.1–66.3 | ||
| Region and stage | |||||||
| Riyadh | Localised | 96 | 93.0 | 87.7–98.4 | 71.6 | 60.8–82.5 | |
| Regional | 138 | 88.8 | 83.5–94.1 | 62.1 | 53.4–70.9 | ||
| Distant | 62 | 58.1 | 45.7–70.5 | 23.1 | 11.8–34.4 | ||
| Unknown | 23 | 94.1 | 83.3–105.0 | 77.2 | 54.9–99.5 | ||
| Makkah | Localised | 142 | 87.9 | 82.2–93.7 | 62.2 | 52.4–72.1 | |
| Regional | 128 | 92.4 | 87.6–97.2 | 59.9 | 50.3–69.5 | ||
| Distant | 55 | 62.4 | 49.1–75.7 | 34.1 | 20.3–47.9 | ||
| Unknown | 36 | 79.3 | 64.8–93.8 | 68.1 | 51.2–85.0 | ||
| Eastern | Localised | 65 | 98.2 | 94.6–101.7 | 75.4 | 63.2–87.6 | |
| Regional | 87 | 86.2 | 78.7–93.7 | 52.7 | 40.9–64.6 | ||
| Distant | 46 | 48.3 | 33.5–63.1 | 7.0 | 0.0–14.8 | ||
| Unknown | 16 | 68.8 | 44.4–93.1 | 58.9 | 32.4–85.5 | ||
| Other | Localised | 72 | 98.1 | 94.5–101.7 | 89.4 | 80.7–98.2 | |
| Regional | 127 | 83.4 | 76.7–90.0 | 62.7 | 53.8–71.5 | ||
| Distant | 58 | 59.9 | 47.4–72.5 | 32.1 | 19.5–44.8 | ||
| Unknown | 62 | 70.5 | 56.5–84.5 | 57.0 | 40.6–73.4 | ||
| Region and stage after imputation§ | |||||||
| Riyadh | Localised | 521 | 93.6 | 88.1–99.1 | 75.1 | 63.7–86.5 | |
| Regional | 740 | 89.5 | 84.1–94.9 | 64.9 | 55.7–74.2 | ||
| Distant | 334 | 59.0 | 46.4–71.6 | 23.9 | 11.9–35.9 | ||
| Makkah | Localised | 781 | 88.9 | 83.1–94.7 | 70.5 | 59.6–81.3 | |
| Regional | 720 | 93.2 | 88.3–98.1 | 62.6 | 52.2–72.9 | ||
| Distant | 304 | 63.7 | 50.1–77.3 | 38.9 | 23.4–54.4 | ||
| Eastern | Localised | 355 | 98.7 | 95.1–100.0 | 79.1 | 66.4–91.7 | |
| Regional | 463 | 87.2 | 79.5–94.8 | 56.2 | 43.6–68.7 | ||
| Distant | 252 | 49.6 | 34.4–64.8 | 7.9 | 0.0–16.6 | ||
| Other | Localised | 511 | 98.7 | 95.1–100.0 | 94.2 | 85–100.0 | |
| Regional | 735 | 84.2 | 77.4–90.9 | 67.8 | 58.2–77.4 | ||
| Distant | 349 | 60.6 | 47.9–73.2 | 34.3 | 20.8–47.7 | ||
§, n is the total from five imputations. CI, confidence interval.
Discussion
Net survival
Age-standardised net survival at one and five years since diagnosis for all Saudi women diagnosed with cervical cancer did not change between 2005–2010 and 2011–2016. Survival was similar for women diagnosed during these two periods in all regions except Makkah, where the 5-year net survival probability increased by 19%. There was a small decline in survival in Riyadh and in the ten peripheral regions.
Five-year net survival (59–60%) for women diagnosed during 2005–2016 was lower than that reported for Saudi Arabia in CONCORD-2 for women diagnosed during 1995–1999 (62.2%; 95% CI: 50.6–73.8%) and 2000–2004 (65.6%; 56.8–74.4%) (2). However, the earlier estimates were flagged as being less reliable due to the very high proportion of women with censored survival times (75.9%). The data submitted for CONCORD-2 only contained follow-up provided by the cancer registry, which was obtained through a mix of active methods (search in patient records) and passive methods (receipt of death notifications and death certificates with a mention of cancer), whereas by linking records to the NIC, survival was only censored for 34% of women in our analysis.
The age-standardised 5-year net survival estimates for cervical cancer in Saudi women were similar to those from other countries in Western Asia reported in CONCORD-3, such as Turkey during 2005–2009 (59.2%; 95% CI: 56.5–61.9%) and 2010–2014 (60.7%; 58.1–63.3%), Jordan during 2010–2014 (56.4%; 48.2–64.6%), Kuwait during 2010–2014 (56.6%; 44.2–69.0%), and Qatar during 2005–2009 (55.5%; 35.3–76.0%) and 2010–2014 (63.5%; 44.2–82.8%). Survival estimates were also similar to those in some Eastern European countries, but lower than in most countries in Western and Northern Europe (1).
A lack of improvement or even a decline in cervical cancer survival has been seen in some countries after the introduction of widespread cervical cancer screening, due to the selective diagnosis and removal of slower-growing precancerous lesions. More aggressive pre-invasive lesions, some of which carry a worse prognosis, may develop and progress to invasive cancer during the intervals between successive screens. However, this is unlikely to explain the lack of improvement in survival in Saudi Arabia, since uptake of Pap smears has been quite low, only about 7.6% of women aged 25–49 having had a Pap smear within the recommended intervals for their age (16).
From 2005–2010 to 2011–2016, the proportion of women diagnosed at a localised stage increased, while the proportion diagnosed at a regional stage declined. However, there was no change in the proportion diagnosed at a distant stage. The probability of surviving up to five years was similar for women diagnosed at a localised or regional stage, while women diagnosed at a distant stage had much lower survival. This may explain the lack of improvement in survival for all stages combined.
The large improvement in survival that was only seen in Makkah cannot be explained by a higher proportion of missing ID numbers or fewer deaths captured by the registry in Makkah during 2011–2016. Nor could it be attributed to improvements in early detection (stage distribution by region did not differ, data not shown). In 2010, the newly founded oncology centre at King Abdullah Medical City became the first specialised cancer centre in the Western Region functioning under the Ministry of Health (MOH), with treatment centres in Jeddah and Makkah. In 2015, it became the first of three centres of integrated oncology and palliative care in Saudi Arabia designated by the European Society for Medical Oncology (ESMO). This led to the availability of more advanced treatment and an increased treatment volume, and may have resulted in more equitable access, efficient referral and timely treatment, translating into improved survival.
A small decrease in survival was seen in Riyadh and the ten peripheral regions. Higher availability of national ID numbers in the SCR records during later years has probably led to improved death ascertainment, but restricting the analysis to women with NIC follow-up did not alter the findings (data not shown). Improvement in completeness and timeliness of the reporting of deaths to the department of civil status may offer a better explanation for this apparent reduction in survival. A fine was imposed in 2015 for failure to notify deaths within 30 days. Electronic death notification was also introduced in MOH hospitals in 2016, and is now being rolled out to other hospitals. In the data with imputed stage, there was no change in the proportion of distant stage, except for Riyadh, where it increased by 4%. This may also explain the observed decrease in survival in that region.
Age-standardised five-year net survival increased slightly between 2005–2010 and 2011–2016 for localised and regional stage, but fell for distant stage. Together with the lack of improvement in survival for all stages combined, this pattern could be attributable to stage migration (17). This possibility is supported by the increase from 7% to 18% in the proportion of women with lymph node extension among women diagnosed at a regional stage (not shown). Stage migration was reported for cervical cancer in a study that compared stage distribution before and after applying the FIGO 2018 criteria, which incorporate advanced imaging findings (18). Improvement in the detection of lymph node extension was the main reason for stage migration in cervical cancer, while the detection of occult metastasis accounted for a small increase in distant stage. The authors did not observe any improvement in survival for women with distant metastasis after upstaging of tumours found to have occult metastasis (18).
Stage-specific unstandardised 1- and 5-year net survival was similar between regions except for the Eastern Region, where 5-year survival for distant stage was substantially (16% to 27%) lower than that in all other regions. However, this was based on only 46 observations. Although the age distribution between regions was similar, women diagnosed at a distant stage in the Eastern Region were on average between 2.1, 3.6 and 5.5 years older than those living in Makkah, Riyadh and the ten peripheral regions, respectively.
A limitation of exploring the missing data mechanism and the process of multiple imputation in the current dataset is the lack of data on comorbidity and treatment (19).
Region of diagnosis may be a more important determinant of survival than region of residence. However, exploring survival by region of diagnosis was not feasible, because fewer women were diagnosed in one of the ten peripheral regions, especially in the older age strata. This could be explored using a larger dataset for a more common cancer.
It has not been possible to examine patterns of survival from cervical cancer in non-Saudi women due to the lack of follow-up data. Non-nationals make up a substantial part of the population and many are long-term residents who are unaccounted for in their countries of origin. Facilitating access to data from the Ministry of Interior on final exit from the country, besides the conventional information on vital status, may enable better understanding of their experience and enable routine estimation of survival for this population.
Conclusions
Cervical cancer survival among Saudi women has remained largely unchanged over the period 2005–2016. Achieving the higher survival seen in other high-income countries will require an increase in the proportion of women who are diagnosed at an early stage, through raising population awareness of early symptoms and signs, and implementation of a high-quality nation-wide screening programme, with a system for recall and referral of women with abnormal findings. It will also be crucial to ensure timely access to high-quality treatment for all women.
Acknowledgments
This work was part of a PhD project for which Dr. Eman Alkhalawi received a scholarship from the government of Saudi Arabia. We thank the staff of the Saudi Cancer Registry and the Cancer Survival Group for their continuous support.
Funding: Eman Alkhalawi was funded by a PhD scholarship from the government of Saudi Arabia.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://ace.amegroups.com/article/view/10.21037/ace-22-2/rc
Data Sharing Statement: Available at https://ace.amegroups.com/article/view/10.21037/ace-22-2/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ace.amegroups.com/article/view/10.21037/ace-22-2/coif). EA was funded by a PhD scholarship from the Saudi Arabian Ministry of Education. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from the London School of Hygiene and Tropical Medicine (#14739, 17 January 2018) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- Ministry of Health, Kingdom of Saudi Arabia. Statistical Yearbook. Chapter 2: Health resources 2020. Available online: https://www.moh.gov.sa/en/Ministry/Statistics/book/Pages/default.aspx
- Al-Ahmadi K, Al-Zahrani A, Al-Ahmadi S. Spatial Accessibility to Cancer Care Facilities in Saudi Arabia. Esri Health GIS Conference; Cambridge, MA, USA; 2014.
- Chen T, Jansen L, Gondos A, et al. Survival of cervical cancer patients in Germany in the early 21st century: a period analysis by age, histology, and stage. Acta Oncol 2012;51:915-21. [Crossref] [PubMed]
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition. Geneva; 2021.
- World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva; 2020.
- Young JJ, Roffers S, Ries L, et al. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute, NIH; 2001.
- Alkhalawi E. Cervical Cancer in Saudi Arabia : Trends and Regional Disparities in Incidence and Survival [PhD thesis]: London School of Hygiene and Tropical Medicine; 2021. doi:
10.17037/PUBS.04661807 .10.17037/PUBS.04661807 - Elandt-Johnson RC, Johnson NL. Survival models and data analysis (Wiley Series in Probability and Mathematical Statistics). Indianapolis: John Wiley & Sons, Inc.; 1980.
- Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics 2012;68:113-20. [Crossref] [PubMed]
- Clerc-Urmès I, Grzebyk M, Hédelin G. Net Survival Estimation with stns. The Stata Journal 2014;14:87-102. [Crossref]
- Brenner H, Gefeller O, Hakulinen T. Period analysis for ‘up-to-date’ cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer 2004;40:326-35. [Crossref] [PubMed]
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307-16. [Crossref] [PubMed]
- Nur U, Shack LG, Rachet B, et al. Modelling relative survival in the presence of incomplete data: a tutorial. Int J Epidemiol 2010;39:118-28. [Crossref] [PubMed]
- So VHT, Channon AA, Ali MM, et al. Uptake of breast and cervical cancer screening in four Gulf Cooperation Council countries. Eur J Cancer Prev 2019;28:451-6. [Crossref] [PubMed]
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985;312:1604-8. [Crossref] [PubMed]
- Grigsby PW, Massad LS, Mutch DG, et al. FIGO 2018 staging criteria for cervical cancer: Impact on stage migration and survival. Gynecol Oncol 2020;157:639-43. [Crossref] [PubMed]
- Di Girolamo C, Walters S, Benitez Majano S, et al. Characteristics of patients with missing information on stage: a population-based study of patients diagnosed with colon, lung or breast cancer in England in 2013. BMC Cancer 2018;18:492. [Crossref] [PubMed]
Cite this article as: Alkhalawi E, Allemani C, Al-Zahrani AS, Coleman MP. Cervical cancer in Saudi Arabia: trends in survival by stage at diagnosis and geographic region. Ann Cancer Epidemiol 2022;6:7.


