
Patient specific factors associated with inpatient hospital length of stay for solid tumor oncology patients: a retrospective cohort study
Introduction
Although most care for oncology is shifted to outpatient settings, hospitalizations for acute illness are inevitable (1,2). For hospitalized cancer patients, the length of stay (LOS) is affected by multiple factors: severity of the illness, comorbidities, type of cancer, and the complexities of its treatments (2,3). LOS is a health system quality metric and is defined as the average number of days patients spend in the hospital. Another measure of LOS, the LOS index (LOSi), is the ratio of actual to expected LOS and is reported as greater than 1 or less than 1, indicating a longer or shorter LOS than expected (4). LOSi varies across health systems, cancer types, and sociodemographic factors of patients (5-7). Michas et al. and others reported the differences in the average LOS for various cancer types from 2008 to 2017 in U.S. hospitals: breast (2.7–4.8 days), prostate (3.5–4.5 days), lung (5.8–6.3 days), and colorectal cancer (7.4–8.1 days) (2,8,9). Recent studies demonstrated that the hospital LOS for cancer patients is also dependent on comorbidities, socioeconomic challenges across different health systems, examination of which is vital to facilitate a timely discharge and a smoother transition to outpatient setting (10-13).
Prior studies from Froedtert Health and the Medical College of Wisconsin report lower survival rates for patients from disadvantaged communities diagnosed with breast and colorectal cancer (14-16). Beyer et al. examined patient-specific sociodemographic factors in Wisconsin. The authors reported the poorest survival among Black/African American and Hispanic/Latina women with breast cancer and lower survival rates among Black/African American patients with colorectal cancer (14). Nattinger et al. examined socioeconomic disparities in mortality rates among breast cancer patients before and after implementation of Medicare Part D (16). Among the pre-Part D beneficiaries, 40.5% of poor women died within five years of diagnosis compared to 20.3% from more affluent communities (16). Unfortunately, some of these inequities related to socioeconomic status (SES) are not limited to the pandemic, but have only widened during the pandemic due to additional financial strain, such as loss of employment leading to delays in routine medical checkups, and unplanned hospitalizations (17-20).
Recently, during the pandemic, throughout the U.S., higher hospitalization rates and poorer outcomes were reported among the underserved: Latinx residents 2.5 times and Blacks or African Americans 2.6 times greater hospitalization rates, and American Indian residents with 1.5 times greater death rates compared to their White counterparts (21-26). Unfortunately, patients with high-risk sociodemographic factors such as the underserved communities, low SES, use of governmental insurance, and other comorbidities such as obesity experienced multiple delays in discharge process due to accompanying impediments (15,16,27-29). These include housing insecurities, transportation barriers, and additional limitations from the accepting facilities related to insurance coverage etc. (30). Also, as the LOS increases, there is an additional risk of hospital acquired infections, which is a major concern for cancer patients who tend to be immunocompromised (31). Accordingly, examination of patient-specific factors for advanced discharge planning has become a higher priority to further identify their home needs to facilitate outpatient referrals. These include home physical therapy and visiting nurse referrals for medication management etc. to facilitate a smoother outpatient transition; especially for those who need additional assistance due to barriers associated with socioeconomic constraints (32-34). These accompanying patient-specific factors and barriers highlight the need for untapped intervention targets; yet only a few studies examined inequities related to hospital LOS based on patient-specific sociodemographic factors (35-39).
During the American Society of Clinical Oncology (ASCO) Quality Training Program (QTP), Stavros Niarchos Quality Improvement Initiative (QI), we conducted a needs assessment for solid tumor oncology patients admitted to inpatient units (40). We identified a longer LOS for patients residing in low SES ZIP Codes in Milwaukee County compared to their high-SES counterparts (7.2 vs. 5.6 days). Based on this previously collected needs assessment data, we examined patient-specific factors by SES, race, and others such as obesity or health insurance payer type to further identify the associated LOS. Although our study planning started in 2019, and not directly linked to the COVID-19 pandemic, the investigators observed longer hospital LOS during the pandemic, especially for patients with socioeconomic constraints. Therefore, we sought to conduct an in-depth evaluation of patient-specific sociodemographic factors and LOSi across different solid cancer diagnoses. We hypothesized that oncology patients with sociodemographic barriers had longer inpatient LOSi than their counterparts. We present the following article in accordance with the STROBE reporting checklist (available at https://ace.amegroups.com/article/view/10.21037/ace-21-15/rc).
Methods
Data source and study sample
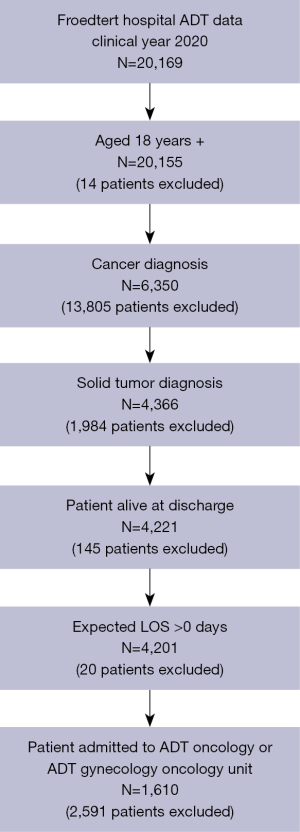
This study evaluated hospital LOS based on institutional census data from EPIC and expected LOS data from the Vizient® Clinical Data Base (CDB) (32,41). The CDB is a healthcare analytics platform that provides data on patient outcomes (e.g., LOS, complication and readmission rates, mortality, etc.) to help hospitals identify performance improvement opportunities. Our study cohort included patients ages 18 and older who were admitted to the inpatient oncology or gynecology oncology units with a diagnosis of solid tumor from 1/1/2020 through 12/31/2020. Solid tumor diagnoses included breast, gastrointestinal (GI), genitourinary (GU), gynecologic (GYN), head and neck (H&N), lung, and melanoma. We excluded patients who died during the hospital stay, had an expected LOS of 0 days, or were missing LOS data. The final study cohort consisted of 1,610 patients (Figure 1).
Study covariates
Based on the data from our own institution and other studies, we selected patients’ sociodemographic factors associated with longer hospital LOS as covariates (14-16). These included indicators of enrollment into Medicare and Medicaid and ecological data at the ZIP Code level (14,16,27) to obtain patients’ demographic and health characteristics, including race, payer type, and BMI from an internal electronic medical record (EMR) database (Table 1). Age group categories included 20–54, 55–64, 65–74, and 75 years and older. Categories of patients’ race/ethnicity included White, Black/African American, and Other. SES was assessed based on patients’ ZIP Codes and categorized as Low, Medium-Low, Medium, Medium-High, High, and Outside Milwaukee County (patients who were not residents of Milwaukee County). Payer types included Medicaid, Medicare, Managed Care and Other/Self-Pay/Unknown. BMI categories included underweight (BMI <18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obese (≥30.0). The data for these variables and clinical data such as solid tumor diagnoses were obtained from an internal clinical electronic Medical Record (EMR-EPIC) database, and International Statistical Classification of Diseases (ICD-10) and merged into the original Vizient® database.
Table 1
| Demographics | Total | Breast | Gastrointestinal | Genitourinary | Gynecologic | Head & neck | Lung | Melanoma | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | P value | Yes | P value | Yes | P value | Yes | P value | Yes | P value | Yes | P value | Yes | P value | ||||||||
| n | 1,610 | 272 | 844 | 343 | 256 | 232 | 553 | 70 | |||||||||||||
| Age, years | 0.007 | 0.005 | <0.001 | 0.52 | <0.001 | 0.51 | 0.75 | ||||||||||||||
| 20–54 | 22.6% [364] | 27.2% [74] | 24.1% [203] | 15.5% [53] | 20.7% [53] | 25.4% [59] | 21.5% [119] | 25.7% [18] | |||||||||||||
| 55–64 | 23.4% [376] | 16.9% [46] | 24.5% [207] | 18.1% [62] | 24.2% [62] | 17.7% [41] | 25.3% [140] | 18.6% [13] | |||||||||||||
| 65–74 | 33.5% [539] | 31.3% [85] | 34.2% [289] | 42.0% [144] | 36.7% [94] | 42.7% [99] | 32.2% [178] | 32.9% [23] | |||||||||||||
| 75 & over | 20.6% [331] | 24.6% [67] | 17.2% [145] | 24.5% [84] | 18.4% [47] | 14.2% [33] | 21.0% [116] | 22.9% [16] | |||||||||||||
| Sex | <0.001 | 0.076 | <0.001 | <0.001 | 0.47 | <0.001 | <0.001 | ||||||||||||||
| Female | 54.3% [875] | 98.2% [267] | 52.3% [441] | 19.0% [65] | 97.7% [250] | 52.2% [121] | 47.9% [265] | 34.3% [24] | |||||||||||||
| Male | 45.7% [735] | 1.8% [5] | 47.7% [403] | 81.0% [278] | 2.3% [6] | 47.8% [111] | 52.1% [288] | 65.7% [46] | |||||||||||||
| Race/ethnicity | 0.96 | 0.22 | 0.002 | 0.9 | 0.48 | 0.89 | <0.001 | ||||||||||||||
| White | 72.7% [1,170] | 72.4% [197] | 70.9% [598] | 80.2% [275] | 73.8% [189] | 75.0% [174] | 72.0% [398] | 92.9% [65] | |||||||||||||
| Black/African American | 21.9% [353] | 22.4% [61] | 23.2% [196] | 16.6% [57] | 21.1% [54] | 19.0% [44] | 22.6% [125] | 0.0% [0] | |||||||||||||
| Other | 5.4% [87] | 5.1% [14] | 5.9% [50] | 3.2% [11] | 5.1% [13] | 6.0% [14] | 5.4% [30] | 7.1% [5] | |||||||||||||
| SES [ZIP code] | 0.009 | 0.041 | 0.082 | 0.88 | 0.046 | 0.075 | 0.006 | ||||||||||||||
| Low | 8.9% [143] | 8.8% [24] | 7.7% [65] | 9.3% [32] | 9.0% [23] | 6.5% [15] | 8.9% [49] | 0.0% [0] | |||||||||||||
| Medium–Low | 11.8% [190] | 9.6% [26] | 13.4% [113] | 8.7% [30] | 10.5% [27] | 9.5% [22] | 14.5% [80] | 7.1% [5] | |||||||||||||
| Medium | 10.9% [175] | 15.4% [42] | 10.9% [92] | 11.4% [39] | 10.2% [26] | 15.5% [36] | 11.6% [64] | 7.1% [5] | |||||||||||||
| Medium–high | 7.4% [119] | 11.0% [30] | 6.2% [52] | 5.8% [20] | 9.0% [23] | 6.5% [15] | 7.4% [41] | 7.1% [5] | |||||||||||||
| High | 4.8% [77] | 4.4% [12] | 4.3% [36] | 7.0% [24] | 4.3% [11] | 6.9% [16] | 5.8% [32] | 1.4% [1] | |||||||||||||
| Not Milwaukee county | 56.0% [902] | 50.7% [138] | 57.1% [482] | 57.7% [198] | 56.6% [145] | 55.2% [128] | 51.7% [286] | 77.1% [54] | |||||||||||||
| Unknown | 0.2% [4] | 0.0% [0] | 0.5% [4] | 0.0% [0] | 0.4% [1] | 0.0% [0] | 0.2% [1] | 0.0% [0] | |||||||||||||
| Payer type | 0.033 | 0.025 | <0.001 | 0.85 | 0.67 | 0.072 | 0.65 | ||||||||||||||
| Medicaid | 11.9% [191] | 8.8% [24] | 11.5% [97] | 7.6% [26] | 12.9% [33] | 11.2% [26] | 14.3% [79] | 7.1% [5] | |||||||||||||
| Medicare | 58.2% [937] | 61.4% [167] | 55.5% [468] | 72.6% [249] | 56.6% [145] | 61.6% [143] | 58.6% [324] | 60.0% [42] | |||||||||||||
| Managed care | 27.1% [436] | 29.0% [79] | 29.5% [249] | 19.0% [65] | 28.1% [72] | 25.0% [58] | 25.0% [138] | 30.0% [21] | |||||||||||||
| Other/self pay/unknown | 2.9% [46] | 0.7% [2] | 3.6% [30] | 0.9% [3] | 2.3% [6] | 2.2% [5] | 2.2% [12] | 2.9% [2] | |||||||||||||
| BMI category | <0.001 | 0.011 | 0.85 | <0.001 | 0.025 | <0.001 | 0.65 | ||||||||||||||
| Underweight | 5.7% [91] | 0.4% [1] | 5.3% [45] | 5.8% [20] | 2.0% [5] | 7.8% [18] | 8.5% [47] | 2.9% [2] | |||||||||||||
| Normal weight | 36.7% [591] | 26.8% [73] | 38.3% [323] | 35.0% [120] | 30.9% [79] | 37.9% [88] | 40.0% [221] | 35.7% [25] | |||||||||||||
| Overweight | 27.5% [443] | 30.9% [84] | 29.7% [251] | 29.2% [100] | 24.6% [63] | 31.5% [73] | 28.6% [158] | 27.1% [19] | |||||||||||||
| Obesity | 29.3% [472] | 41.5% [113] | 26.1% [220] | 29.2% [100] | 39.1% [100] | 21.6% [50] | 22.1% [122] | 34.3% [24] | |||||||||||||
| Unknown | 0.8% [13] | 0.4% [1] | 0.6% [5] | 0.9% [3] | 3.5% [9] | 1.3% [3] | 0.9% [5] | 0.0% [0] | |||||||||||||
Data presented as % [n]. GI, gastrointestinal; GU, genitourinary; H&N, head and neck. LOSi, length of stay index; SES, socioeconomic; BMI, body mass index.
Study outcome
We identified the LOSi as the study outcome, calculated by dividing the observed LOS by the expected LOS, values obtained from the Vizient® Clinical Data Base (41). Observed LOS was defined as the number of days between a patient’s date of admission and date of discharge. Vizient® used data from 2 years of admissions from 176 academic medical centers to build a predictive model for LOS based on demographic and clinical characteristics present at admission (e.g., age, gender, comorbidities, etc.) (42). This predictive model was applied to our cohort to obtain expected LOS for each admission. A LOSi of greater than 1 denoted a LOS that was longer than expected, whereas a LOSi of less than 1 denoted a LOS shorter than expected (19). The LOSi ratio was used to compare the LOSi for the categories of the main predictors to a reference category (LOSi for Black/African American patients compared to White patients).
Statistical analyses
Poisson regression models with robust standard errors were used to estimate the LOSi and LOSi ratio (43). The outcome variable was the LOS, in days, while the log-transformed value of the expected LOS was used as an offset in the model in addition to the covariates of interest. We constructed separate models for each type of cancer since the risk profile for morbidity and mortality differs significantly for each. We examined the LOSi estimates for each category of the predictor, P values comparing if the LOSi was statistically significantly different from 1, and 95% confidence intervals (CI). We examined the LOSi ratio estimates for each category of the predictor compared to the reference group, P values statistically comparing the LOSi to the reference group LOSi, and 95% CI. Statistical analysis was performed using R version 4.0.3.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was exempt from formal IRB approval by institutional ethics board of Medical College of Wisconsin. Individual consent for this retrospective analysis was waived.
Results
A total of 1,610 patients with a solid tumor diagnosis admitted to hematology and oncology units were identified (Table 1). Oncological diagnoses were defined as a patient being diagnosed with at least one of the following solid tumor malignancies: breast (16.9%), GI (52.4%), GU (21.3%), GYN (15.9%), H&N (14.4%), lung (34.3), and melanoma (4.3%). Approximately 45.1% of the sample was diagnosed with more than one type of cancer up to 5 different cancer diagnoses. Many patients were 65–74 years (33.5%), female (54.3%), identified as White (72.7%), and had a normal weight (36.7%). Although most patients resided outside Milwaukee County, the majority who did live in Milwaukee County had medium-low SES (11.8%). Additionally, most patients used Medicare (58.2%) as their primary insurance type.
Tables 2-4 include univariate and multivariate Poisson regression models for LOSi and LOSi ratio. Among all patients with a solid tumor diagnosis (Table 2), patients with medium-low SES had a higher LOSi and LOSi ratio for both univariate and multivariate analyses (LOSi =1.20, CI: 1.03–1.39, P=0.02; LOSi ratio =1.21, CI: 1.03–1.42, P=0.02; aLOSi ratio =1.30, CI: 1.04–1.62, P=0.02). Among patients with breast cancer, significant differences in the LOSi, univariate LOSi ratio, and multivariate LOSi ratio were seen across different social characteristics (Table 3). The LOSi was higher for patients who identified as Black (LOSi =1.24, CI: 1.07–1.45, P=0.01), had medium-low SES (LOSi =1.46, CI: 1.08–1.99, P=0.02), used Medicaid as their primary payer type (LOSi =1.40, CI: 1.13–1.74, P=0.00), and were underweight (LOSi =1.66, CI: 1.66–1.66, P=0.00) or overweight (LOSi =1.23, CI: 1.05–1.43, P=0.01). In univariate analyses, the LOSi ratio was significantly higher for patients who had medium-low SES (LOSi ratio =1.46, CI: 1.04–2.03, P=0.03), used Medicaid (LOSi ratio=1.51, CI: 1.17–1.96, P=0.00) or Medicare (LOSi ratio =1.22, CI: 1.00–1.48, P=0.05), and were underweight (LOSi ratio=1.79, CI: 1.55–2.06, P=0.00) or overweight (LOSi ratio=1.32, CI: 1.07–1.63, P=0.01). When controlling for race/ethnicity, SES, payer type, and BMI, the LOSi ratio was significantly higher for patients who used Medicare (aLOSi ratio =1.22, CI: 1.00–1.49, P=0.05) and were overweight (aLOSi ratio =1.28, CI: 1.03–1.60, P=0.03). Among patients who were diagnosed with head and neck cancer, patients who identified as Black had a lower LOSi and LOSi ratio for both univariate and multivariate analyses (LOSi =0.77, CI: 0.61–0.97, P=0.02; LOSi ratio =0.73, CI: 0.55–0.95, P=0.02; aLOSi ratio =0.67, CI: 0.46–0.99, P=0.04). No other significant differences were noted among the other solid tumor cancer types.
Table 2
| Demographics | Mean | LOSi^ | LOSi ratio^ | LOSi ratio^^ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (N=1,610) | Mean observed LOS [days] | Mean expected LOS [days] | LOSi | 95% CI | P | LOSi ratio | 95% CI | P | aLOSi ratio | 95% CI | P | ||||
| Race/ethnicity | |||||||||||||||
| White | 1,170 | 6.94 | 6.75 | 1.03 | 0.97–1.09 | 0.34 | – | – | – | – | – | – | |||
| Black | 353 | 7.34 | 6.84 | 1.07 | 0.97–1.19 | 0.18 | 1.05 | 0.93–1.18 | 0.46 | 0.90 | 0.74–1.09 | 0.27 | |||
| Other | 87 | 6.15 | 6.50 | 0.95 | 0.81–1.10 | 0.47 | 0.92 | 0.78–1.08 | 0.32 | 0.85 | 0.70–1.02 | 0.07 | |||
| SES [ZIP code] | |||||||||||||||
| Low SES | 143 | 7.31 | 6.56 | 1.11 | 0.95–1.30 | 0.17 | 1.12 | 0.95–1.33 | 0.17 | 1.21 | 0.96–1.53 | 0.10 | |||
| Medium-Low SES | 190 | 8.38 | 6.99 | 1.20 | 1.03–1.39 | 0.02 | 1.21 | 1.03–1.42 | 0.02 | 1.30 | 1.04–1.62 | 0.02 | |||
| Medium SES | 175 | 7.13 | 6.51 | 1.10 | 0.94–1.28 | 0.25 | 1.11 | 0.94–1.31 | 0.23 | 1.16 | 0.96–1.41 | 0.12 | |||
| Medium-High SES | 119 | 6.71 | 6.98 | 0.96 | 0.84–1.10 | 0.57 | 0.97 | 0.84–1.13 | 0.69 | 0.99 | 0.85–1.16 | 0.90 | |||
| High SES | 77 | 7.14 | 7.32 | 0.98 | 0.82–1.16 | 0.79 | 0.99 | 0.82–1.19 | 0.88 | 1.00 | 0.83–1.21 | 0.98 | |||
| Other [not Milwaukee county] | 902 | 6.65 | 6.71 | 0.99 | 0.93–1.05 | 0.77 | – | – | – | – | – | – | |||
| Payer [health insurance] | |||||||||||||||
| Medicaid | 191 | 6.89 | 6.68 | 1.03 | 0.90–1.18 | 0.65 | 1.04 | 0.89–1.23 | 0.61 | 1.00 | 0.84–1.19 | 1.00 | |||
| Medicare | 937 | 7.30 | 6.99 | 1.04 | 0.98–1.11 | 0.16 | 1.06 | 0.94–1.18 | 0.33 | 1.05 | 0.94–1.18 | 0.38 | |||
| Managed care | 436 | 6.26 | 6.34 | 0.99 | 0.90–1.09 | 0.80 | – | – | – | – | – | – | |||
| Other/self-pay/unknown | 46 | 7.80 | 6.39 | 1.22 | 0.92–1.61 | 0.16 | 1.24 | 0.92–1.66 | 0.16 | 1.25 | 0.94–1.66 | 0.12 | |||
| BMI | |||||||||||||||
| Underweight | 91 | 9.34 | 8.41 | 1.11 | 0.88–1.40 | 0.38 | 1.10 | 0.86–1.40 | 0.46 | 1.10 | 0.87–1.40 | 0.42 | |||
| Normal weight | 591 | 6.89 | 6.80 | 1.01 | 0.94–1.09 | 0.73 | – | – | – | – | – | – | |||
| Overweight | 443 | 7.19 | 6.63 | 1.08 | 0.99–1.19 | 0.08 | 1.07 | 0.95–1.20 | 0.26 | 1.07 | 0.95–1.20 | 0.30 | |||
| Obese | 472 | 6.58 | 6.54 | 1.01 | 0.93–1.09 | 0.88 | 0.99 | 0.89–1.11 | 0.91 | 0.99 | 0.88–1.11 | 0.84 | |||
^, Univariate Poisson Regression Models with robust standard errors; ^^, Multivariate Poisson Regression Models with robust standard errors; controlled for all other predictors. LOSi, length of stay index; SES, socioeconomic; BMI, body mass index.
Table 3
| Demographics | Mean | LOSi^ | LOSi ratio^ | LOSi ratio^^ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (N=272) | Mean observed LOS (days) | Mean expected LOS [days] | LOSi | 95% CI | P | LOSi ratio | 95% CI | P | aLOSi ratio | 95% CI | P | ||||
| Race/ethnicity | |||||||||||||||
| White | 197 | 6.62 | 6.34 | 1.04 | 0.92–1.19 | 0.50 | – | – | – | – | – | – | |||
| Black | 61 | 7.82 | 6.29 | 1.24 | 1.07–1.45 | 0.01 | 1.19 | 0.97–1.45 | 0.09 | 0.92 | 0.61–1.39 | 0.68 | |||
| Other | 14 | 6.50 | 5.40 | 1.20 | 0.77–1.89 | 0.42 | 1.15 | 0.72–1.84 | 0.56 | 1.10 | 0.68–1.80 | 0.70 | |||
| SES [ZIP code] | |||||||||||||||
| Low SES | 24 | 7.25 | 5.81 | 1.25 | 0.91–1.72 | 0.18 | 1.24 | 0.88–1.76 | 0.22 | 1.24 | 0.86–1.80 | 0.25 | |||
| Medium-Low SES | 26 | 10.04 | 6.87 | 1.46 | 1.08–1.99 | 0.02 | 1.46 | 1.04–2.03 | 0.03 | 1.43 | 0.78–2.61 | 0.25 | |||
| Medium SES | 42 | 7.19 | 6.42 | 1.12 | 0.87–1.44 | 0.37 | 1.11 | 0.84–1.48 | 0.45 | 1.07 | 0.73–1.56 | 0.74 | |||
| Medium-High SES | 30 | 6.50 | 5.37 | 1.21 | 0.94–1.56 | 0.14 | 1.20 | 0.90–1.60 | 0.20 | 1.18 | 0.87–1.61 | 0.28 | |||
| High SES | 12 | 9.33 | 11.21 | 0.83 | 0.57–1.21 | 0.34 | 0.83 | 0.56–1.23 | 0.35 | 0.83 | 0.51–1.35 | 0.45 | |||
| Other [not Milwaukee county] | 138 | 6.01 | 5.98 | 1.00 | 0.88–1.15 | 0.95 | – | – | – | – | – | – | |||
| Payer [health insurance] | |||||||||||||||
| Medicaid | 24 | 7.38 | 5.26 | 1.40 | 1.13–1.74 | 0.00 | 1.51 | 1.17–1.96 | 0.00 | 1.30 | 0.95–1.78 | 0.10 | |||
| Medicare | 167 | 7.78 | 6.90 | 1.13 | 0.98–1.29 | 0.09 | 1.22 | 1.00–1.48 | 0.05 | 1.22 | 1.00–1.49 | 0.05 | |||
| Managed care | 79 | 4.95 | 5.35 | 0.93 | 0.80–1.07 | 0.29 | – | – | – | – | – | – | |||
| Other/self-pay/unknown | 2 | 3.00 | 3.67 | 0.82 | 0.41–1.62 | 0.56 | 0.88 | 0.44–1.78 | 0.73 | 0.77 | 0.31–1.88 | 0.56 | |||
| BMI | |||||||||||||||
| Underweight | 1 | 9.00 | 5.42 | 1.66 | 1.66–1.66 | 0.00 | 1.79 | 1.55–2.06 | 0.00 | 1.29 | 0.91–1.83 | 0.10 | |||
| Normal weight | 73 | 5.79 | 6.26 | 0.93 | 0.80–1.07 | 0.29 | – | – | – | – | – | – | |||
| Overweight | 84 | 6.88 | 5.61 | 1.23 | 1.05–1.43 | 0.01 | 1.32 | 1.07–1.63 | 0.01 | 1.28 | 1.03–1.60 | 0.03 | |||
| Obese | 113 | 7.60 | 6.77 | 1.12 | 0.94–1.34 | 0.20 | 1.21 | 0.97–1.52 | 0.10 | 1.19 | 0.92–1.56 | 0.19 | |||
^, Univariate Poisson Regression Models with robust standard errors; ^^, Multivariate Poisson Regression Models with robust standard errors; controlled for all other predictors. LOSi, length of stay index; SES, socioeconomic; BMI, body mass index.
Table 4
| Patient demographics | Mean | LOSi^ | LOSi ratio^ | aLOSi ratio^^ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (N=232) | Mean observed LOS (days) | Mean expected LOS (days) | LOSi | 95% CI | P | LOSi ratio | 95% CI | P | LOSi ratio | 95% CI | P | ||||
| Race/ethnicity | |||||||||||||||
| White | 174 | 7.67 | 7.24 | 1.06 | 0.92–1.22 | 0.44 | – | – | – | – | – | – | |||
| Black | 44 | 5.91 | 7.69 | 0.77 | 0.61–0.97 | 0.02 | 0.73 | 0.55–0.95 | 0.02 | 0.67 | 0.46–0.99 | 0.04 | |||
| Other | 14 | 6.64 | 6.67 | 1.00 | 0.65–1.52 | 0.99 | 0.94 | 0.60–1.47 | 0.79 | 0.91 | 0.55–1.51 | 0.71 | |||
| SES [zip code] | |||||||||||||||
| Low SES | 15 | 9.60 | 8.88 | 1.08 | 0.65–1.80 | 0.76 | 1.01 | 0.59–1.73 | 0.97 | 1.22 | 0.61–2.41 | 0.58 | |||
| Medium-Low SES | 22 | 5.91 | 6.68 | 0.89 | 0.67–1.17 | 0.40 | 0.83 | 0.59–1.15 | 0.26 | 0.98 | 0.66–1.45 | 0.92 | |||
| Medium SES | 36 | 6.42 | 7.37 | 0.87 | 0.67–1.13 | 0.30 | 0.81 | 0.59–1.11 | 0.20 | 0.90 | 0.66–1.24 | 0.53 | |||
| Medium-High SES | 15 | 7.20 | 7.22 | 1.00 | 0.73–1.36 | 0.99 | 0.93 | 0.66–1.32 | 0.69 | 1.08 | 0.74–1.58 | 0.70 | |||
| High SES | 16 | 7.44 | 9.15 | 0.81 | 0.55–1.21 | 0.31 | 0.76 | 0.49–1.17 | 0.21 | 0.73 | 0.45–1.17 | 0.19 | |||
| Other [not Milwaukee county] | 128 | 7.46 | 6.97 | 1.07 | 0.90–1.27 | 0.43 | – | – | – | – | – | – | |||
| Payer [health insurance] | |||||||||||||||
| Medicaid | 26 | 7.65 | 7.86 | 0.97 | 0.78–1.21 | 0.81 | 1.06 | 0.78–1.44 | 0.71 | 1.09 | 0.76–1.58 | 0.63 | |||
| Medicare | 143 | 7.52 | 7.40 | 1.02 | 0.86–1.20 | 0.85 | 1.11 | 0.84–1.46 | 0.47 | 1.21 | 0.91–1.60 | 0.19 | |||
| Managed care | 58 | 6.34 | 6.91 | 0.92 | 0.74–1.14 | 0.44 | – | – | – | – | – | – | |||
| Other/self-pay/unknown | 5 | 8.80 | 5.69 | 1.55 | 0.49–4.88 | 0.46 | 1.69 | 0.52–5.42 | 0.38 | 1.76 | 0.55–5.63 | 0.34 | |||
| BMI | |||||||||||||||
| Underweight | 18 | 8.83 | 8.97 | 0.98 | 0.66–1.46 | 0.94 | 1.02 | 0.66–1.57 | 0.93 | 1.06 | 0.67–1.67 | 0.81 | |||
| Normal weight | 88 | 7.26 | 7.53 | 0.96 | 0.81–1.15 | 0.68 | – | – | – | – | – | – | |||
| Overweight | 73 | 7.36 | 6.52 | 1.13 | 0.88–1.44 | 0.33 | 1.17 | 0.87–1.57 | 0.30 | 1.25 | 0.93–1.66 | 0.14 | |||
| Obese | 50 | 6.86 | 7.53 | 0.91 | 0.70–1.18 | 0.48 | 0.94 | 0.69–1.29 | 0.72 | 1.09 | 0.80–1.48 | 0.59 | |||
^, Univariate Poisson Regression Models with robust standard errors; ^^, Multivariate Poisson Regression Models with robust standard errors; controlled for all other predictors. LOSi, length of stay index; SES, socioeconomic; BMI, body mass index.
Discussion
Our study addressed patient-specific differences in LOS by race, SES, payer type, and BMI for hospitalized patients with a solid tumor cancer diagnosis in a combined analysis and separately by the disease type. Our results demonstrated increases in LOSi, and LOSi ratio both in the univariate (P=0.02) and multivariate analyses (P=0.02) for solid tumor oncology patients from medium-low SES groups. We also noted significant findings among specific disease subtypes: among breast cancer patients, those who identified as Black, had medium-low SES, used Medicaid, and were underweight or overweight had longer LOS compared to their counterparts, some of which persisted both in the univariate and multivariate models. However, among H&N cancer patients, Black patients had a shorter LOS, highlighting the sociodemographic differences contributing to extended hospital LOS among various solid tumor oncology patients. As many of our patients require outpatient appointments for follow-up care, we believe that addressing patients’ sociodemographics at the time of hospital admission is vital to facilitate interventions while transitioning to the outpatient setting.
Previous studies evaluating LOS among breast cancer patients examined the type of breast cancer surgery (lumpectomy vs. mastectomy) with LOS, but studies examining the LOS related to sociodemographic constraints for hospitalized patients on active cancer treatment are limited (38,39,44). As most of oncology care is offered in the outpatient setting, hospitalizations have significantly lowered in the past few years for breast cancer patients. However, our study demonstrated the patient-specific factors that contributed to a longer LOS. This, in turn, indicates an opportunity to identify and address these factors upfront at the time of admission to facilitate a timely discharge process.
Few other studies explored inpatient LOS for oncology patients with other tumor types. In contrast to our study results, a German study by Schneider et al. examined the differences in the number and duration of hospital stays and reported that lung cancer patients had frequent hospitalization (3.3 times higher) compared to breast, colon and prostate cancer patients and for a long duration (35 days) (3). In a UK-based study, Aravani et al. evaluated LOS after surgical resection for colorectal cancer during 1998–2010 (n=240,873). Although the overall LOS was ten days after the surgical resection, there was a decline in the LOS from 11 days in 1998 to seven days in 2010 (8). Nevertheless, neither one of these studies focused on differences in race, SES, payer type, or BMI, which are important in clinical practice as patient-specific barriers that may delay the continuity of cancer care if not addressed timely. In a study from Brazil, Silva et al. examined the number of hospitalizations within the first year of outpatient cancer treatment, LOS and patients’ demographics and characteristics during the years 2010–2014 (9). The authors reported hospitalizations among 34% of patients, with a median LOS being six days, with lower admissions rates for female patients [odds ratio (OR): 0.84; 95% CI: 0.82–0.86] and shorter length hospital stay. In addition to the regional differences in LOS, patients with colorectal cancer had a higher frequency of hospitalizations (OR: 4.42; 95% CI: 4.27–∞ and LOS (average ratio, AR: 1.37; 95% CI: 1.35–1.40) (21).
Only a limited number of retrospective studies attempted to address sociodemographic factors and the associated indicators of health inequities associated with hospital LOS for oncology patients (38,39). Our study showed no increased LOS based on race, SES, payer type, or BMI for lung, gastrointestinal, genitourinary, or gynecologic cancers. In contrast to our study results, in a study by Naik et al., a nationwide inpatient sample of patients diagnosed with prostate cancer reported a longer inpatient LOS for Black patients compared to White patients regardless of other demographics and patient characteristics (38). Naik et al. also evaluated the LOS for cervical cancer patients and reported a shorter LOS for White patients compared to Black/African-Americans (β=0.41, P<0.0005) (39). Although the exact reasons for these differences are unclear, the authors reported possible association with comorbidities, and treatment at low volume hospitals which may have caused a protracted course of hospitalization among African American patients (39).
Our study is unique in identifying patient-specific factors related to hospital LOS among inpatient oncology patients in the city of Milwaukee, WI, USA and the neighboring counties, given the statewide inequities among cancer patients from vulnerable communities (34). In addition, in our observation, throughout the U.S., the COVID-19 pandemic had a direct influence on cancer care, leading to delays in treatment, and infrequent medical checkups leading to higher rates of hospitalizations, and an overall compromise in the overall health of cancer patients (35,45,46). Additionally, patients with coexisting comorbidities, low SES, barriers to transportation, and suboptimal health insurance also suffered from multiple hospitalizations, emergency room visits, and readmissions (20,33). Based on this information, it is imperative that we identify patient-related factors in facilitating the admission to discharge process by involving the appropriate teams, i.e., transportation, home nurse, etc.
Several strengths of this study include exploring multiple patient-specific factors including BMI for inpatient cancer patients that are restricting the hospital discharge process, providing opportunities to develop patient-specific care-delivery models, which set examples and foundations for future studies. Unlike prior studies, the analyses were adjusted for expected LOS, which captured age, gender, comorbidities, diagnoses, and procedures. Although our results vary compared to previous reports, we believe that unique barriers related to geographical aspects in Milwaukee, WI, USA, such as poverty among the underserved, may cause multiple hurdles and delays in the cancer treatment leading to hospitalizations and longer stay. Our study had a few limitations including it was inclusive of all inpatient oncology admissions during 1/1/2020 through 12/31/2020 and being limited to oncology patients in a geographic location, which may limit generalizability. While this study assessed SES based on patients’ ZIP codes, the authors acknowledge the possible inaccuracies in the source of information on EMR.
Conclusions
This study shows that certain patient-specific factors such as race, SES, primary payer, and BMI contribute to inpatient LOS for patients with solid tumor oncological diagnoses. Although not unique to the pandemic, examination of patient’s needs and barriers at the time of admission became more relevant during the pandemic; generating appropriate referrals and to facilitate a safe and timely discharge. Healthcare systems may benefit by using these patient-specific factors to develop care-delivery models to reduce inpatient LOS and provide a personalized approach to addressing health inequities for patients with cancer.
Acknowledgments
Funding: The work was supported by American Society of Clinical Oncology (ASCO) Niarchos Foundation Grant and, Faculty Vitality Award, Medical College of Wisconsin (Department of Medicine, Division of Hematology-Oncology).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://ace.amegroups.com/article/view/10.21037/ace-21-15/rc
Peer Review File: Available at https://ace.amegroups.com/article/view/10.21037/ace-21-15/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ace.amegroups.com/article/view/10.21037/ace-21-15/coif). SK reports that this work was supported by American Society of Clinical Oncology (ASCO) Niarchos Foundation Grant and, Faculty Vitality Award, Medical College of Wisconsin (Department of Medicine, Division of Hematology-Oncology). The ASCO grant is a quality improvement (QI) grant only with no direct transaction of funds. The Faculty Vitality Award provided funds to hire a coordinator for the QI projects. There are no other direct benefits involved. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was exempt from formal IRB approval by institutional ethics board of Medical College of Wisconsin. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martos-Benítez FD, Soler-Morejón CD, Lara-Ponce KX, et al. Critically ill patients with cancer: A clinical perspective. World J Clin Oncol 2020;11:809-35. [Crossref] [PubMed]
- Michas F. Inpatient average length of stay in U.S. hospitals by cancer type 2008-2017, Statista, USA. Aug 26, 2020. Available online: https://www.statista.com/statistics/325181/average-hospital-stay-in-the-us-per-inpatient-case-by-cancer-type/
- Schneider N, Dreier M, Amelung VE, et al. Hospital stay frequency and duration of patients with advanced cancer diseases - differences between the most frequent tumour diagnoses: a secondary data analysis. Eur J Cancer Care (Engl) 2007;16:172-7. [Crossref] [PubMed]
- Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA 2010;303:2141-7. [Crossref] [PubMed]
- Bein T, Hackner K, Zou T, et al. Socioeconomic status, severity of disease and level of family members' care in adult surgical intensive care patients: the prospective ECSSTASI study. Intensive Care Med 2012;38:612-9. [Crossref] [PubMed]
- Cupp J, Culakova E, Poniewierski MS, et al. Analysis of Factors Associated With In-hospital Mortality in Lung Cancer Chemotherapy Patients With Neutropenia. Clin Lung Cancer 2018;19:e163-9. [Crossref] [PubMed]
- D'Almeida CA, Peres WAF, de Pinho NB, et al. Prevalence of Malnutrition in Older Hospitalized Cancer Patients: A Multicenter and Multiregional Study. J Nutr Health Aging 2020;24:166-71. [Crossref] [PubMed]
- Aravani A, Samy EF, Thomas JD, et al. A retrospective observational study of length of stay in hospital after colorectal cancer surgery in England (1998-2010). Medicine (Baltimore) 2016;95:e5064. [Crossref] [PubMed]
- Feliciana Silva F, Macedo da Silva Bonfante G, Reis IA, et al. Hospitalizations and length of stay of cancer patients: A cohort study in the Brazilian Public Health System. PLoS One 2020;15:e0233293. [Crossref] [PubMed]
- Nuruzzaman N, Broadwin M, Kourouma K, et al. Making the social determinants of health a routine part of medical care. J Health Care Poor Underserved 2015;26:321-7. [Crossref] [PubMed]
- Jabbarpour YM, Raney LE. Bridging Transitions of Care From Hospital to Community on the Foundation of Integrated and Collaborative Care. Focus (Am Psychiatr Publ) 2017;15:306-15. [Crossref] [PubMed]
- Gruneir A, Bronskill SE, Maxwell CJ, et al. The association between multimorbidity and hospitalization is modified by individual demographics and physician continuity of care: a retrospective cohort study. BMC Health Serv Res 2016;16:154. [Crossref] [PubMed]
- Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff (Millwood) 2014;33:778-85. [Crossref] [PubMed]
- Beyer KM, Zhou Y, Matthews K, et al. Breast and Colorectal Cancer Survival Disparities in Southeastern Wisconsin. WMJ 2016;115:17-21. [PubMed]
- Beyer KMM, Laud PW, Zhou Y, et al. Housing discrimination and racial cancer disparities among the 100 largest US metropolitan areas. Cancer 2019;125:3818-27. [Crossref] [PubMed]
- Nattinger AB, Wozniak EM, McGinley EL, et al. Socioeconomic Disparities in Mortality Among Women With Incident Breast Cancer Before and After Implementation of Medicare Part D. Med Care 2017;55:463-9. [Crossref] [PubMed]
- Hertz-Palmor N, Moore TM, Gothelf D, et al. Association among income loss, financial strain and depressive symptoms during COVID-19: Evidence from two longitudinal studies. J Affect Disord 2021;291:1-8. [Crossref] [PubMed]
- Gualano MR, Lo Moro G, Voglino G, et al. Effects of Covid-19 Lockdown on Mental Health and Sleep Disturbances in Italy. Int J Environ Res Public Health 2020;17:4779. [Crossref] [PubMed]
- Zachary Z, Brianna F, Brianna L, et al. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract 2020;14:210-6. [Crossref] [PubMed]
- Aboueshia M, Hussein MH, Attia AS, et al. Cancer and COVID-19: analysis of patient outcomes. Future Oncol 2021;17:3499-510. [Crossref] [PubMed]
- Services WDoH. COVID-19: Racial and Ethnic Disparities. 2021. Available online: https://www.dhs.wisconsin.gov/covid-19/disparities.htm
- Getachew Y, Zephyrin L, Abrams MK, et al. Beyond the Case Count: The Wide-Ranging Disparities of COVID-19 in the United States. 2020. Available online: https://www.commonwealthfund.org/publications/2020/sep/beyond-case-count-disparities-covid-19-united-states
- Prevention CCfDCa. Risk for COVID-19 infection, hosptialization, and death by race/ethnicity. CDC Webpage 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
- Printz C. Research shows racial disparity, mortality data for patients with cancer and COVID-19. Cancer 2021;127:333. [Crossref] [PubMed]
- Kumar V, Grant M, Martin KA. Risk, Racial Disparity, and Outcomes Among Patients With Cancer and COVID-19 Infection. JAMA Oncol 2021;7:1065. [Crossref] [PubMed]
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907-18. [Crossref] [PubMed]
- Bellini B, Cresci B, Cosentino C, et al. Obesity as a risk factor for hospitalization in COronaVirus Disease-19 (COVID-19) patients: Analysis of the Tuscany regional database. Nutr Metab Cardiovasc Dis 2021;31:769-73. [Crossref] [PubMed]
- Calder PC, Carr AC, Gombart AF, et al. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020;12:1181. [Crossref]
- Søgaard M, Thomsen RW, Bossen KS, et al. The impact of comorbidity on cancer survival: a review. Clin Epidemiol 2013;5:3-29. [Crossref] [PubMed]
- Ye H, Lee S, Kim H. Effects of Neighborhood Characteristics on Length of Inpatient Stay: Findings from the U.S. National Data. Soc Work Res 2016;40:117-26. [Crossref] [PubMed]
- Cornejo-Juárez P, Vilar-Compte D, García-Horton A, et al. Hospital-acquired infections at an oncological intensive care cancer unit: differences between solid and hematological cancer patients. BMC Infect Dis 2016;16:274. [Crossref] [PubMed]
- Richards M, Anderson M, Carter P, et al. The impact of the COVID-19 pandemic on cancer care. Nat Cancer 2020;1:565-7. [Crossref]
- Atalla E, Kalligeros M, Giampaolo G, et al. Readmissions among patients with COVID-19. Int J Clin Pract 2021;75:e13700. [Crossref] [PubMed]
- Hayda J. Wisconsin's COVID-19 death disparity is 3rd worst in America. Is segregation to blame? WUWM 897 Milwaukee's NPR 2020. Available online: https://www.wuwm.com/race-ethnicity/2020-05-29/wisconsins-covid-19-death-disparity-is-3rd-worst-in-america-is-segregation-to-blame
- Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med 2020;18:270. [Crossref] [PubMed]
- Lapidus N, Zhou X, Carrat F, et al. Biased and unbiased estimation of the average length of stay in intensive care units in the Covid-19 pandemic. Ann Intensive Care 2020;10:135. [Crossref] [PubMed]
- Singu S, Acharya A, Challagundla K, et al. Impact of Social Determinants of Health on the Emerging COVID-19 Pandemic in the United States. Front Public Health 2020;8:406. [Crossref] [PubMed]
- Naik G, Akinyemiju T. Disparities in hospitalization outcomes among African-American and White prostate cancer patients. Cancer Epidemiol 2017;46:73-9. [Crossref] [PubMed]
- Naik G, Mukherjee A, Akinyemiju T, et al. Hospitalization outcomes and racial disparities in cervical cancer patients: An analysis of the national inpatient sample data from 2002 to 2014. Cancer Epidemiol 2019;63:101620. [Crossref] [PubMed]
- Kamaraju S, Mohan M, Wright T, et al. Addressing the Disparities and the Factors Related to Prolonged Inpatient Length of Stay for Solid Tumor Oncology Patients During the COVID-19 Pandemic: A Narrative Review. Journal of Radiology and Oncology 2021;5:46-53. [Crossref]
-
Vizient Clinical Data Base - Committee V. 2021 Risk Adjustment Methodology. 2021.
- Cameron AC, Trivedi PK. Microeconometrics Using Stata. The Stata News 2008;23:1-6.
- Gümüş M, Satıcı Ö, Ülger BV, et al. Factors Affecting the Postsurgical Length of Hospital Stay in Patients with Breast Cancer. J Breast Health 2015;11:128-31. [Crossref] [PubMed]
- DeGroff A, Miller J, Sharma K, et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Prev Med 2021;151:106559. [Crossref] [PubMed]
- Riera R, Bagattini ÂM, Pacheco RL, et al. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob Oncol 2021;7:311-23. [Crossref] [PubMed]
Cite this article as: Kamaraju S, Canales B, Wright T, Charlson J, Wetzel T, Cadman J, Szabo A, Williams J, Power S, Campbell G. Patient specific factors associated with inpatient hospital length of stay for solid tumor oncology patients: a retrospective cohort study. Ann Cancer Epidemiol 2022;6:5.


