
Survival trends for primary liver cancer, 1995–2009: analysis of individual data for 578,740 patients from 187 population-based registries in 36 countries (CONCORD-2)
Introduction
Primary liver cancer is the fifth most common cancer world-wide, and the second most common cause of cancer death, with an estimated 841,100 new cases and 781,500 deaths each year (1). More than 70% of cases and deaths arise in males. Hepatocellular carcinoma (HCC) accounts for 60–80% of invasive malignancies of the liver (2). It is estimated that 80% of HCC cases are secondary to chronic infection with hepatitis B or C (3). Aflatoxin contamination of cereals and peanuts is estimated to cause up to 28% of cases in sub-Saharan Africa, Southeast Asia, and China (4). In high-income countries, where incidence rates are lower, important risk factors are chronic hepatitis C infection, alcohol-induced cirrhosis (5), and increasingly, liver disease linked with diabetes and obesity (6).
Most other primary malignancies of the liver are cholangiocarcinomas [10–40% of cases (2)], arising in the intrahepatic bile ducts. In South-east Asia, particularly Thailand, infestation with the liver flukes Opisthorchis viverrini and Clonorchis sinensis is an endemic risk factor (7). Risk factors in other countries include primary sclerosing cholangitis (8), cholelithiasis (9) and hepatitis C infection (10), but cholangiocarcinoma has also been associated with smoking (11) and obesity (12).
A large proportion of the global burden of liver cancer, therefore, is potentially preventable through reductions in exposure to risk, particularly chronic viral infection. However, over 80% of HCC occur in sub-Saharan Africa and Asia (3). Vaccination against hepatitis viruses for primary prevention can be difficult in low- and middle-income countries with limited infrastructure (13,14), although such a programme was successfully introduced in the Gambia (15). The global burden of incidence is thus likely to remain high for the foreseeable future. Global surveillance of survival is required, both to identify international variation in outcomes (16) and to identify modifiable prognostic factors in a given country, such as health-seeking behaviour, screening, access to services, early diagnosis and treatment, and health system organization (17).
Trends in population-based survival enable the overall effectiveness of the health system in each country to be monitored. Five-year net survival from liver cancer is very low (10–20%) in both developed and developing countries (18,19). Survival for patients whose cholangiocarcinoma is localised and who receive a transplant and chemoradiation can be as high as 68% at 5 years (20), while it can be as high as 75% for those with very early HCC (21); however, only a small proportion of patients are diagnosed sufficiently early for surgery and transplantation to be viable, even in developed countries. Patients with intra- or extra-hepatic metastases fare much less well, with five-year survival typically below 10% (21).
We present international comparisons of trends in population-based net survival up to five years after diagnosis of primary cancer of the liver among adults diagnosed during 1995–2009 in 36 countries that were included in the CONCORD-2 study (19).
Methods
Methods of data acquisition, quality control and analysis for the CONCORD-2 study, and ethical approval, have been described (19). Data were submitted by 243 population-based cancer registries in 60 countries on 1,005,032 adults (aged 15–99 years) diagnosed with their first, primary, invasive, malignant neoplasm of the liver or intrahepatic bile ducts [International Classification of Diseases for Oncology, third revision (ICD-O-3) (22), C22.0 and C22.1] between 1995 and 2009. After exclusion of 22,175 records during data quality control, 982,857 patients were eligible for inclusion in analyses.
The liver is a common site for metastatic spread from cancer in other organs, so we only included primary, invasive, malignant tumours of the liver (behaviour code /3) for which the registry provided evidence of histological or cytological confirmation of the diagnosis, or a specific morphology code (i.e., excluding ICD-O-3 8000–8005), irrespective of the basis of diagnosis. We also included patients whose cancer was diagnosed with the specific tumour marker alpha-fetoprotein (usually >200 ng/mL serum) and coded as HCC, not otherwise specified (ICD-O-3 morphology 8170), according to guidelines from the European Network of Cancer Registries (ENCR) (23). We excluded data from registries for which the liver cancer survival estimates had been flagged as less reliable in CONCORD-2 (19). We also excluded patients whose tumour was registered only from a death certificate (DCO), or solely at autopsy.
We defined two main morphological groups: HCC (ICD-O-3 8170–8175) and cholangiocarcinoma (ICD-O-3 8050, 8140–8141, 8160–8161, 8260, 8440, 8480–8500, 8570–8572) (24).
Five-year net survival was estimated with the non-parametric Pohar-Perme estimator (25) using the Stata (26) program stns (27). Net survival deploys life tables of all-cause mortality rates in the general population by age, sex and year, to correct for the effect of the wide international variations in non-cancer mortality. Life tables were constructed from death and population counts by single year of age or five-year age group, sex, race/ethnicity (where possible) and calendar year or period, for the territory of each participating registry or country (28). The classical cohort approach was used to estimate survival for patients diagnosed during 1995–2000 and 2001–2003, because at least five years of follow-up for vital status were available for all these patients by 31 December 2009. We estimated survival for patients diagnosed during 2004–2009 with the complete approach (29), because not all patients had been followed up for five years. We also estimated five-year survival conditional on survival to the end of the first year after diagnosis, as a surrogate for survival in patients with local or regional disease, since patients with advanced disease are unlikely to survive more than one year. The calendar periods were chosen to match the availability of data on stage from 2001, and changes in the data collection processes for coding SEER Summary Stage 2000 from 2004 (30).
We estimated net survival for each of five age groups, and used the International Cancer Survival Standard (ICSS) weights (15–44 years, 0.07; 45–54 years, 0.12; 55–64 years, 0.23; 65–74 years, 0.29; 75–99 years, 0.29) to produce age-standardised survival estimates for all ages combined (31). Age-specific survival was only estimated if data for at least 50 patients were available for analysis, and at least 10 deaths had been observed. If a survival estimate could not be obtained for a particular age group, the data for two adjacent groups were combined, and the analysis repeated. The pooled estimate was then used for both age groups in age-standardization.
Funnel plots (32) were adopted for graphical presentation, in preference to the conventional ranked bar charts, in order to identify countries with unexpectedly high or low survival, given the precision of the estimate. A random effects model (33), fitted by restricted maximum likelihood estimation, adjusted for the precision of each estimate, was used to estimate the mean and variance of the distribution of five-year survival estimates for all countries included in each analysis. The analysis was performed on the complementary log-log scale (34), with 5% ‘winsorisation’ (32) to reduce inflation of the variance. We use this pooled estimate as the target in the funnel plot, for purely descriptive purposes. The standard error of each estimate and the standard deviation between countries, derived from the random effects model, were used to construct the control limits of the funnel plot; estimates outside the 95.0% or 99.8% control limits are at least 1.96 and 3.09 standard deviations from the target, respectively (34).
Since none of the age-standardised survival estimates for 2004–2009 exceeded the upper 95% control limit in the funnel plot, we changed the ‘target’ or benchmark, to the pooled survival estimate for patients diagnosed during 1995–2000. This was done in order to help identify countries or registries in which the age-standardised 5-year net survival for patients diagnosed during 2004–2009 was higher than for patients diagnosed 10 years earlier. A similar approach was used to identify age-specific survival estimates for 2004–2009 that were higher than the corresponding pooled estimate for patients diagnosed during 1995–2000.
Results
Patients
Of the 982,857 patients eligible for inclusion in CONCORD-2, we excluded 166,557 (16.9%) patients from 56 registries in 24 countries for which the survival estimates were considered less reliable (19), or for which fewer than 50 patients were available for analysis in each calendar period, leaving 816,300 patients (Figure 1). We excluded a further 41,650 patients (4.2% of those eligible) whose tumour was registered from a death certificate only, or at post-mortem, or for other reasons (Table 1), and 195,910 patients (19.9% of those eligible) with no evidence of microscopic verification or a specific morphology code, including a code derived from the alpha-fetoprotein level (23). We included 578,740 patients (58.9% of eligible patients) from 187 registries in 36 countries in survival analyses. Age-standardised estimates of five-year net survival were available for 28 of the 36 countries (Table 2).
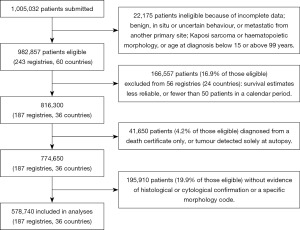
Table 1
| Region | Eligible patients | Exclusions (%)† | After exclusions | Data quality indicators (%)†† | Patients included in analysis††† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DCO or PM | Other | MV | Non–specific morphology | Lost to follow-up | Censored | All periods (1995–2009) | 1995–2000 | 2001–2003 | 2004–2009 | ||||
| America (Central and South) | |||||||||||||
| Colombia (Cali) | 750 | 12.0 | 1.3 | 650 | 67.7 | 31.5 | 0.0 | 5.1 | 458 | 126 | 92 | 240 | |
| America (North) | |||||||||||||
| Canada* | 22,479 | 4.7 | 1.3 | 21,124 | 53.1 | 0.0 | 0.0 | 0.0 | 11,902 | 3,774 | 2,247 | 5,881 | |
| US registries | 197,772 | 5.9 | 3.0 | 180,218 | 74.6 | 0.0 | 0.0 | <0.1 | 140,046 | 41,026 | 27,208 | 71,812 | |
| Asia | |||||||||||||
| Chinese registries | 33,387 | 2.7 | <0.1 | 32,482 | 25.6 | 71.2 | 1.4 | <0.1 | 10,569 | 710 | 1,715 | 8,144 | |
| Indonesia (Jakarta) | 305 | 1.3 | 0.0 | 301 | 21.6 | 71.8 | 0.0 | 0.0 | 85 | 85 | |||
| Japanese registries | 27,759 | 20.5 | 0.2 | 22,025 | 31.9 | 10.2 | 0.0 | 1.2 | 19,882 | 2,186 | 1,401 | 16,295 | |
| Korea* | 184,632 | <0.1 | 0.5 | 183,659 | 28.1 | 13.3 | 0.0 | 0.0 | 160,125 | 44,510 | 34,081 | 81,534 | |
| Malaysia (Penang) | 986 | 10.3 | 0.7 | 877 | 65.3 | 9.0 | 0.0 | 0.0 | 814 | 214 | 126 | 474 | |
| Mongolia* | 6,701 | 0.0 | 5.1 | 6,358 | 6.6 | <0.1 | 15.5 | 0.0 | 422 | 422 | |||
| Taiwan* | 133,641 | 0.0 | 0.2 | 133,440 | 41.9 | 25.7 | 0.0 | 0.0 | 99,383 | 14,945 | 21,482 | 62,956 | |
| Thai registries | 15,590 | 6.3 | 0.1 | 14,600 | 11.0 | 84.0 | 0.0 | 18.3 | 1,614 | 341 | 414 | 859 | |
| Turkey (Izmir) | 1,399 | 6.2 | 1.6 | 1,290 | 57.1 | 0.8 | 0.0 | 21.2 | 736 | 183 | 147 | 406 | |
| Europe | |||||||||||||
| Austria* | 10,088 | 0.3 | 6.8 | 9,368 | 88.6 | 3.7 | 0.0 | 0.0 | 9,184 | 3,198 | 2,053 | 3,933 | |
| Belgium* | 3,079 | <0.1 | 0.8 | 3,050 | 87.3 | 3.6 | 1.4 | 0.0 | 2,958 | 2,958 | |||
| Denmark* | 4,069 | 0.8 | 0.0 | 4,035 | 84.6 | 20.8 | <0.1 | 0.0 | 3,519 | 1,288 | 734 | 1,497 | |
| Estonia* | 1,016 | 13.7 | <0.1 | 876 | 69.5 | 7.5 | 0.0 | 0.0 | 609 | 274 | 121 | 214 | |
| Finland* | 4,817 | 14.2 | <0.1 | 4,129 | 80.6 | 26.7 | <0.1 | 0.0 | 3,434 | 1,122 | 611 | 1,701 | |
| French registries | 9,025 | <0.1 | 0.3 | 8,996 | 59.7 | 16.9 | 0.9 | 0.6 | 6,500 | 3,118 | 1,970 | 1,412 | |
| German registries | 8,151 | 10.9 | 1.7 | 7,119 | 71.8 | 1.6 | 0.3 | 0.0 | 7,034 | 1,423 | 1,185 | 4,426 | |
| Ireland* | 1,716 | 5.4 | 3.4 | 1,564 | 51.5 | 3.8 | 0.0 | 0.0 | 811 | 195 | 143 | 473 | |
| Italian registries | 45,542 | 5.4 | 1.2 | 42,614 | 43.5 | 42.8 | 0.6 | 0.2 | 24,401 | 9,245 | 6,090 | 9,066 | |
| Malta* | 82 | 13.4 | 6.1 | 66 | 100.0 | 25.8 | 0.0 | 0.0 | 66 | 66 | |||
| Netherlands* | 4,940 | 2.9 | 0.7 | 4,764 | 74.1 | 25.8 | 0.5 | 0.0 | 3,557 | 1,215 | 711 | 1,631 | |
| Norway* | 1,851 | 3.3 | 0.0 | 1,789 | 81.6 | 12.9 | 0.1 | 0.0 | 1,583 | 546 | 322 | 715 | |
| Polish registries | 14,673 | 12.1 | 0.4 | 12,833 | 43.2 | 1.3 | 0.2 | 0.0 | 5,553 | 713 | 1,368 | 3,472 | |
| Portugal* | 3,768 | 0.7 | 2.5 | 3,647 | 85.6 | 11.4 | <0.1 | 0.3 | 3,285 | 542 | 891 | 1,852 | |
| Romania (Cluj) | 362 | 55.2 | 0.3 | 161 | 82.0 | 0.6 | 0.0 | 0.0 | 142 | 142 | |||
| Russia (Arkhangelsk) | 245 | 11.4 | 4.1 | 210 | 56.2 | 4.3 | 1.0 | 0.0 | 119 | 51 | 68 | ||
| Slovakia* | 165 | 17.6 | 0.0 | 136 | 91.9 | 8.8 | 0.0 | 0.0 | 125 | 125 | |||
| Slovenia* | 1,868 | 11.2 | 0.1 | 1,658 | 63.8 | 35.1 | <0.1 | 0.0 | 1,086 | 388 | 228 | 470 | |
| Spanish registries | 13,157 | 7.2 | 0.8 | 12,105 | 47.4 | 22.7 | 0.2 | <0.1 | 7,811 | 2,864 | 1,765 | 3,182 | |
| Sweden* | 7,543 | 0.0 | 0.0 | 7,543 | 92.9 | 10.8 | 0.2 | 0.0 | 7,044 | 2,961 | 1,419 | 2,664 | |
| Swiss registries | 4,360 | 4.9 | 1.7 | 4,072 | 59.8 | 16.0 | 0.8 | 0.9 | 3,095 | 1,020 | 658 | 1,417 | |
| United Kingdom* | 36,779 | 7.1 | <0.1 | 34,152 | 46.0 | 5.7 | <0.1 | <0.1 | 29,912 | 8,751 | 5,843 | 15,318 | |
| Oceania | |||||||||||||
| Australian registries | 11,150 | 4.0 | 1.1 | 10,583 | 56.5 | 16.7 | 0.0 | 1.0 | 8,845 | 2,804 | 2,002 | 4,039 | |
| New Zealand* | 2,453 | 11.9 | 0.2 | 2,156 | 60.1 | 6.4 | 0.0 | 0.0 | 2,031 | 521 | 393 | 1,117 | |
| Total | 816,300 | – | – | 774,650 | – | – | – | – | 578,740 | 150,328 | 117,471 | 310,941 | |
*, data with 100% coverage of the national population; †, DCO: patients registered from a death certificate only (DCO), or whose tumour was detected solely at autopsy. Other: vital status or sex unknown; invalid sequence of dates; inconsistency of sex-site, site-morphology, age-site, age-morphology, or age-site-morphology. ††, MV: microscopically verified. Non-specific morphology: ICD–O–3 morphology code in the range 8000–8005. Censored: for patients diagnosed during 1995–2004, alive with less than five years of follow-up. †††, patients with microscopic verification as the basis of diagnosis, or with a specific morphology code (see ‘Methods’).
Table 2
| Region | 1995–2000 | 2001–2003 | 2004–2009 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age-standardized | Unstandardized | No. | Age-standardized | Unstandardized | No. | Age-standardized | Unstandardized | ||||||||||||
| NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | |||||||||
| America (Central and South) | ||||||||||||||||||||
| Colombia (Cali) | 126 | 3.9 | 0.0–7.9 | 92 | 7.4 | 1.2–13.7 | 240 | 3.4 | 0.0–7.2 | |||||||||||
| America (North) | ||||||||||||||||||||
| Canada* | 3,774 | 14.1 | 12.9–15.2 | 14.6 | 13.3–15.8 | 2,247 | 18.8 | 17.1–20.5 | 19.2 | 17.5–21.0 | 5,881 | 21.3 | 19.6–23.1 | 22.3 | 20.5–24.1 | |||||
| US registries | 41,026 | 9.6 | 9.2–9.9 | 9.6 | 9.3–9.9 | 27,208 | 13.5 | 13.0–13.9 | 14.0 | 13.5–14.4 | 71,812 | 16.3 | 15.9–16.8 | 17.0 | 16.5–17.5 | |||||
| Asia | ||||||||||||||||||||
| Chinese registries | 710 | 2.7 | 1.6–3.8 | 3.6 | 2.1–5.0 | 1,715 | 20.4 | 17.6–23.2 | 19.8 | 17.7–21.8 | 8,144 | 19.5 | 18.0–21.0 | 20.1 | 18.9–21.4 | |||||
| Indonesia (Jakarta) | 85 | 1.9 | 0.0–5.8 | |||||||||||||||||
| Japanese registries | 2,186 | 27.9 | 25.9–29.8 | 28.6 | 26.5–30.7 | 1,401 | 26.4 | 24.0–28.8 | 25.5 | 23.0–28.0 | 16,295 | 23.7 | 22.6–24.7 | 23.0 | 22.1–23.9 | |||||
| Korea* | 44,510 | 12.3 | 11.9–12.8 | 14.5 | 14.1–14.8 | 34,081 | 16.4 | 15.8–16.9 | 19.4 | 19.0–19.9 | 81,534 | 21.2 | 20.6–21.7 | 24.4 | 24.0–24.9 | |||||
| Malaysia (Penang) | 214 | 10.8 | 6.4–15.3 | 126 | 13.2 | 7.0–19.3 | 474 | 15.1 | 10.6–19.7 | 16.1 | 11.5–20.7 | |||||||||
| Mongolia* | 422 | 8.5 | 3.1–13.9 | 11.3 | 2.0–20.7 | |||||||||||||||
| Taiwan* | 14,945 | 26.6 | 25.7–27.6 | 27.4 | 26.7–28.2 | 21,482 | 21.1 | 20.5–21.8 | 22.6 | 22.0–23.2 | 62,956 | 22.7 | 22.1–23.2 | 23.9 | 23.3–24.4 | |||||
| Thai registries | 341 | 19.6 | 13.4–25.8 | 18.5 | 12.1–24.8 | 414 | 5.2 | 3.1–7.3 | 6.9 | 3.7–10.0 | 859 | 4.4 | 2.3–6.5 | 5.7 | 3.2–8.1 | |||||
| Turkey (Izmir) | 183 | 19.1 | 9.9–28.4 | 147 | 27.8 | 18.6–37.0 | 406 | 21.5 | 15.7–27.2 | |||||||||||
| Europe | ||||||||||||||||||||
| Austria* | 3,198 | 8.5 | 7.4–9.6 | 7.6 | 6.6–8.6 | 2,053 | 12.0 | 10.4–13.6 | 11.3 | 9.8–12.8 | 3,933 | 12.8 | 11.2–14.4 | 11.9 | 10.4–13.4 | |||||
| Belgium* | 2,958 | 20.5 | 17.8–23.1 | 20.3 | 17.6–23.1 | |||||||||||||||
| Denmark* | 1,288 | 2.7 | 1.8–3.5 | 2.5 | 1.6–3.4 | 734 | 3.8 | 2.5–5.1 | 3.6 | 2.2–5.1 | 1,497 | 6.7 | 4.8–8.6 | 6.2 | 3.9–8.4 | |||||
| Estonia* | 274 | 5.0 | 2.2–7.8 | 121 | 8.4 | 3.2–13.6 | 214 | 7.5 | 2.8–12.1 | |||||||||||
| Finland* | 1,122 | 7.7 | 6.0–9.5 | 7.5 | 5.6–9.3 | 611 | 7.2 | 4.9–9.4 | 1,701 | 8.4 | 6.2–10.6 | 7.8 | 5.5–10.1 | |||||||
| French registries | 3,118 | 12.9 | 11.5–14.2 | 12.2 | 10. 9–13.5 | 1,970 | 14.9 | 13.1–16.6 | 14.3 | 12.6–16.0 | 1,412 | 18.6 | 16.2–21.0 | 18.4 | 15.9–21.0 | |||||
| German registries | 1,423 | 7.4 | 6.0–8.8 | 6.6 | 5.2–8.1 | 1,185 | 8.5 | 6.9–10.1 | 8.2 | 6.4–9.9 | 4,426 | 14.7 | 12.9–16.5 | 13.5 | 11.8–15.2 | |||||
| Ireland* | 195 | 9.0 | 4.7–13.3 | 143 | 17.9 | 11.1–24.7 | 473 | 17.0 | 12.9–21.1 | 16.1 | 11.0–21.2 | |||||||||
| Italian registries | 9,245 | 15.1 | 14.2–15.9 | 14.5 | 13.7–15.3 | 6,090 | 19.3 | 18.1–20.5 | 18.2 | 17.1–19.3 | 9,066 | 21.7 | 20.4–23.0 | 19.9 | 18.6–21.1 | |||||
| Malta* | 66 | 2.2 | 0.0–5.6 | |||||||||||||||||
| Netherlands* | 1,215 | 8.3 | 6.7–10.0 | 8.3 | 6.6–10.0 | 711 | 11.8 | 9.5–14.1 | 12.3 | 9.7–14.9 | 1,631 | 12.0 | 9.5–14.4 | 12.1 | 9.4–14.9 | |||||
| Norway* | 546 | 6.1 | 4.2–8.1 | 5.2 | 3.2–7.2 | 322 | 7.2 | 4.6–9.8 | 6.7 | 3.9–9.6 | 715 | 11.0 | 7.9–14.1 | 10.3 | 6.9–13.6 | |||||
| Polish registries | 713 | 8.3 | 5.7–10.9 | 8.1 | 5.9–10.4 | 1,368 | 10.3 | 8.4–12.1 | 10.2 | 8.5–12.0 | 3,472 | 9.3 | 7.6–11.0 | 9.6 | 8.0–11.3 | |||||
| Portugal* | 542 | 10.4 | 7.9–12.9 | 10.1 | 7.4–12.8 | 891 | 13.0 | 10.6–15.4 | 13.3 | 10.8–15.7 | 1,852 | 16.3 | 13.8–18.8 | 15.8 | 13.4–18.2 | |||||
| Romania (Cluj) | 142 | 2.4 | 0.0–5.8 | |||||||||||||||||
| Russia (Arkhangelsk) | 51 | 2.2 | 0.0–5.5 | 68 | 8.3 | 0.9–15.6 | ||||||||||||||
| Slovakia* | 125 | 6.6 | 2.0–11.1 | |||||||||||||||||
| Slovenia* | 388 | 3.3 | 2.0–4.7 | 3.7 | 1.7–5.6 | 228 | 5.6 | 2.5–8.8 | 470 | 6.0 | 3.3–8.7 | 4.8 | 0.5–9.1 | |||||||
| Spanish registries | 2,864 | 12.5 | 11.1–13.8 | 11.8 | 10.5–13.1 | 1,765 | 15.6 | 13.8–17.5 | 14.6 | 12.8–16.4 | 3,182 | 18.0 | 16.1–20.0 | 17.4 | 15.4–19.4 | |||||
| Sweden* | 2,961 | 5.6 | 4.6–6.6 | 4.4 | 3.6–5.2 | 1,419 | 5.8 | 4.6–7.1 | 5.1 | 3.8–6.4 | 2,664 | 12.9 | 11.0–14.7 | 10.8 | 9.0–12.6 | |||||
| Swiss registries | 1,020 | 10.5 | 8.7–12.3 | 9.8 | 7.8–11.8 | 658 | 13.7 | 11.1–16.4 | 12.9 | 10.1–15.7 | 1,417 | 15.2 | 12.5–17.9 | 14.8 | 11.9–17.7 | |||||
| United Kingdom* | 8,751 | 6.4 | 5.8–7.0 | 5.4 | 4.9–5.9 | 5,843 | 8.3 | 7.5–9.2 | 7.0 | 6.3–7.7 | 15,318 | 9.3 | 8.4–10.1 | 7.8 | 7.0–8.5 | |||||
| Oceania | ||||||||||||||||||||
| Australian registries | 2,804 | 14.2 | 12.8–15.5 | 13.9 | 12.6–15.3 | 2,002 | 14.1 | 12.5–15.7 | 13.8 | 12.2–15.5 | 4,039 | 13.9 | 12.1–15.7 | 13.7 | 11.9–15.6 | |||||
| New Zealand* | 521 | 12.2 | 9.2–15.1 | 13.5 | 10.3–16.7 | 393 | 12.4 | 9.5–15.4 | 14.5 | 10.8–18.2 | 1,117 | 16.5 | 13.2–19.9 | 17.0 | 13.5–20.5 | |||||
| Combined estimate†† | 150,328 | 11.0 | 8.4–13.5 | 10.4 | 8.1–12.7 | 117,471 | 13.3 | 10.9–15.7 | 12.6 | 10.4–14.9 | 310,941 | 14.8 | 12.8–16.8 | 13.2 | 11.0–15.5 | |||||
*, data with 100% coverage of the national population; †, microscopically verified (see text); ††, estimated with a random effects model (see text).
Data quality
The proportion of tumours registered as a DCO or without microscopic verification varied widely (Table 1). DCO registrations exceeded 10% in 12 of the 36 countries. In China, Indonesia, Mongolia, Thailand and Poland, more than 50% of patients were excluded for lack of microscopic verification or a specific morphology code (not shown). In Thailand, Denmark, Poland and Sweden, 20% or more of cholangiocarcinomas were coded as arising in the liver (C22.0), rather than the intrahepatic bile ducts, while in Malaysia, 14% of HCC were coded as arising in the intrahepatic bile ducts (C22.1; Table S1).
The number of patients with data on stage at diagnosis was too small to enable international comparison of age-standardised net survival by stage.
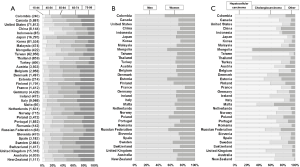
Age, sex and histological group
Patients in low- and middle-income countries were generally younger than in European countries and Japan (Figure 2A). Most patients diagnosed during 2004–2009 were male (median proportion 69.4%, Figure 2B). HCC was more common than cholangiocarcinoma (median 70.4% and 19.4%, respectively; Figure 2C). HCC represented 84.0–89.7% of liver cancers in Taiwan, Japan, and Korea, while cholangiocarcinoma represented 67.4% of liver cancers in Thailand and 43.9% in the UK (Table S1).
Five-year net survival of patients diagnosed in 2004–2009
For all liver cancers combined, the pooled estimate of age-standardised five-year net survival in 28 countries for patients diagnosed during 2004–2009 was 14.8% (range 4.4–23.7%; Table 2). Survival was much lower than the pooled estimate for the same period in Denmark (6.7%), Slovenia (6.0%), and Thailand (4.4%; Figure 3A). None of the estimates exceeded the upper limit of the funnel plot.
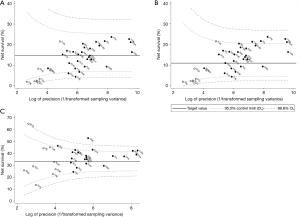
Five-year survival for patients diagnosed during 2004–2009 in Canada, Italy, Japan, Taiwan, and Korea (21.2–23.7%) was higher than the upper 95% control limit around the 1995–2000 benchmark (11.0%) (Figure 3B).
Age-standardised five-year conditional survival for patients diagnosed during 2004–2009 who had survived for at least one year varied from 24.4% to 52.7% (Table 3). In New Zealand, China, Canada, Taiwan and Korea, conditional survival for 2004–2009 (42.0–52.7%) was above the upper 95% control limit around the 1995–2000 benchmark (33.2%; Figure 3C).
Table 3
| Region | All primary liver cancers | Hepatocellular carcinoma | Cholangiocarcinoma | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-year survival | Conditional survival | 5-year survival | Conditional survival | 5-year survival | Conditional survival | ||||||||||||
| NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | ||||||
| America (North) | |||||||||||||||||
| Canada* | 21.3 | 19.6–23.1 | 43.0 | 39.4–46.6 | 25.4 | 23.3–27.5 | 46.1 | 42.1–50.1 | 8.1 | 5.6–10.6 | 25.1 | 17.6–32.6 | |||||
| US registries | 16.3 | 15.9–16.8 | 38.8 | 37.7–40.0 | 17.7 | 17.2–18.3 | 40.0 | 38.7–41.3 | 8.8 | 7.9–9.8 | 27.3 | 24.3–30.3 | |||||
| Asia | |||||||||||||||||
| Chinese registries | 19.5 | 18.0–21.0 | 52.7 | 48.7–56.7 | 21.3 | 19.4–23.1 | 51.9 | 47.3–56.5 | 16.0 | 11.5–20.5 | |||||||
| Japanese registries | 23.7 | 22.6–24.7 | 37.4 | 35.9–39.0 | 25.5 | 24.4–26.7 | 38.4 | 36.7–40.1 | 10.9 | 8.8–13.0 | |||||||
| Korea* | 21.2 | 20.6–21.7 | 42.0 | 40.7–43.2 | 24.1 | 23.4–24.8 | 43.8 | 42.4–45.3 | 9.5 | 8.5–10.4 | 31.9 | 28.5–35.4 | |||||
| Malaysia (Penang) | 15.1 | 10.6–19.7 | 10.6 | 6.9–14.3 | |||||||||||||
| Mongolia* | 8.5 | 3.1–13.9 | |||||||||||||||
| Taiwan* | 22.7 | 22.1–23.2 | 42.3 | 41.2–43.4 | 24.0 | 23.4–24.7 | 42.8 | 41.7–44.0 | 8.3 | 7.1–9.6 | 31.9 | 27.2–36.5 | |||||
| Thai registries | 4.4 | 2.3–6.5 | 3.7 | 1.9–5.6 | |||||||||||||
| Europe | |||||||||||||||||
| Austria* | 12.8 | 11.2–14.4 | 35.1 | 30.9–39.3 | 14.6 | 12.5–16.7 | 37.0 | 32.0–41.9 | 5.7 | 3.4–8.0 | |||||||
| Belgium* | 20.5 | 17.8–23.1 | 40.6 | 35.3–45.9 | 23.0 | 19.8–26.2 | 44.4 | 38.4–50.4 | 14.4 | ||||||||
| Denmark* | 6.7 | 4.8–8.6 | 8.1 | 5.5–10.7 | |||||||||||||
| Finland* | 8.4 | 6.2–10.6 | 24.4 | 18.5–30.3 | 11.3 | 8.0–14.7 | 3.7 | 1.6–5.9 | |||||||||
| French registries | 18.6 | 16.2–21.0 | 34.9 | 30.5–39.3 | 20.5 | 17.6–23.4 | 35.9 | 31.0–40.8 | |||||||||
| German registries | 14.7 | 12.9–16.5 | 36.7 | 32.4–41.0 | 16.3 | 14.0–18.6 | 38.9 | 33.7–44.0 | 10.8 | 7.8–13.9 | |||||||
| Ireland* | 17.0 | 12.9–21.1 | |||||||||||||||
| Italian registries | 21.7 | 20.4–23.0 | 37.7 | 35.6–39.8 | 24.0 | 22.5–25.5 | 39.6 | 37.3–42.0 | 7.9 | 5.6–10.2 | |||||||
| Netherlands* | 12.0 | 9.5–14.4 | 32.2 | 25.8–38.6 | 13.6 | 10.5–16.7 | 33.0 | 25.7–40.3 | 5.6 | 2.8–8.4 | |||||||
| Norway* | 11.0 | 7.9–14.1 | 14.0 | 9.8–18.1 | |||||||||||||
| Polish registries | 9.3 | 7.6–11.0 | 27.8 | 22.4–33.2 | 9.4 | 7.0–11.9 | 25.2 | 18.7–31.6 | 8.8 | 6.4–11.3 | |||||||
| Portugal* | 16.3 | 13.8–18.8 | 40.9 | 34.7–47.0 | 16.8 | 13.7–19.8 | 38.3 | 31.7–45.0 | 13.3 | 9.5–17.2 | |||||||
| Slovenia* | 6.0 | 3.3–8.7 | 7.7 | 4.4–10.9 | |||||||||||||
| Spanish registries | 18.0 | 16.1–20.0 | 38.0 | 33.7–42.3 | 19.3 | 17.0–21.7 | 38.0 | 33.3–42.7 | 11.4 | 7.8–14.9 | |||||||
| Sweden* | 12.9 | 11.0–14.7 | 42.7 | 37.0–48.3 | 17.3 | 14.9–19.7 | 46.5 | 40.0–52.9 | 5.4 | 3.4–7.5 | |||||||
| Swiss registries | 15.2 | 12.5–17.9 | 35.6 | 29.4–41.9 | 18.2 | 15.1–21.4 | 40.2 | 33.4–47.0 | |||||||||
| United Kingdom* | 9.3 | 8.4–10.1 | 29.4 | 26.8–32.1 | 12.5 | 11.2–13.8 | 35.2 | 31.3–39.1 | 5.3 | 4.3–6.3 | 19.8 | 16.2–23.4 | |||||
| Oceania | |||||||||||||||||
| Australian registries | 13.9 | 12.1–15.7 | 31.3 | 27.1–35.6 | 16.9 | 14.6–19.2 | 36.5 | 31.5–41.5 | 7.2 | 4.9–9.6 | 16.7 | 11.1–22.4 | |||||
| New Zealand* | 16.5 | 13.2–19.9 | 46.1 | 37.3–54.9 | 20.3 | 15.9–24.7 | 8.7 | 5.0–12.4 | |||||||||
| Combined estimate††† | 14.8 | 12.8–16.8 | 37.6 | 34.9–40.4 | 17.4 | 15.2–19.5 | 39.7 | 37.2–42.3 | 8.4 | 7.0–9.9 | 25.6 | 20.6–30.6 | |||||
*, data with 100% coverage of the national population; †, five-year survival conditional on survival to the end of the first year after diagnosis; ††, microscopically verified (see text); †††, estimated with a random effects model (see text).
Hepatocellular carcinoma
The pooled estimate of age-standardised five-year net survival for patients diagnosed during 2004–2009 was 17.4% (range 7.7–25.5%; Table 3). Survival in Slovenia (7.7%) and Denmark was lower than the pooled estimate (8.1%; Figure 4A). None of the estimates exceeded the upper 95% control limit of the funnel plot.
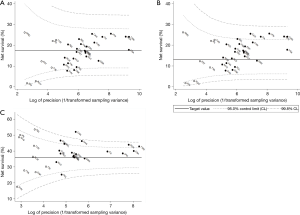
Five-year survival for patients diagnosed during 2004–2009 was higher than the upper 95% control limit for 1995–2000 in Canada, Italy, Japan, Taiwan and Korea (24.0–25.5%; Figure 4B), suggesting progress from the levels ten years earlier.
Conditional survival for patients diagnosed during 2004–2009 was higher than the 95% control limits for 1995–2000 in China, Sweden, Belgium, Canada, Korea and Taiwan (42.8–51.9%; Figure 4C), also suggesting progress in these countries.
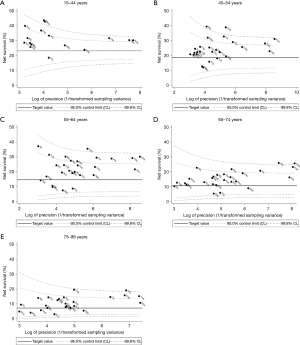
Five-year net survival is generally lower in older patients. The pooled estimates of five-year net survival for patients diagnosed during 2004–2009 aged 15–44, 45–54, 55–64, 65–74 and 75–99 years were 30.6%, 24.6%, 21.4%, 15.8% and 10.2%, respectively (Table 4).
Table 4
| Region | 15–44 years | 45–54 years | 55–64 years | 65–74 years | 75–99 years | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | NS (%) | 95% CI | No. | NS (%) | 95% CI | No. | NS (%) | 95% CI | No. | NS (%) | 95% CI | No. | NS (%) | 95% CI | |||||
| America (North) | |||||||||||||||||||
| Canada* | 177 | 42.8 | 33.4–52.2 | 746 | 38.9 | 34.0–43.8 | 1,145 | 35.4 | 31.3–39.6 | 1,206 | 21.7 | 17.5–26.0 | 1,055 | 11.3 | 7.3–15.4 | ||||
| US registries | 2,273 | 31.6 | 29.2–34.1 | 11,720 | 22.9 | 21.8–24.0 | 17,104 | 21.6 | 20.5–22.6 | 13,152 | 16.2 | 15.1–17.3 | 12,507 | 10.8 | 9.6–11.9 | ||||
| Asia | |||||||||||||||||||
| Chinese registries | 707 | 23.8 | 20.0–27.7 | 1,538 | 25.7 | 22.9–28.4 | 1,313 | 23.2 | 20.0–26.3 | 1,336 | 19.0 | 15.8–22.2 | 721 | 19.6 | 14.7–24.5 | ||||
| Japanese registries | 198 | 33.4 | 24.7–42.1 | 803 | 29.0 | 25.2–32.8 | 3,087 | 28.8 | 26.7–31.0 | 5,692 | 25.9 | 24.3–27.5 | 4,441 | 19.2 | 17.3–21.1 | ||||
| Korea* | 6,646 | 30.3 | 28.8–31.8 | 18,418 | 31.1 | 30.1–32.1 | 19,688 | 30.1 | 29.0–31.1 | 15,510 | 23.2 | 22.0–24.4 | 6,289 | 15.8 | 13.9–17.7 | ||||
| Malaysia (Penang) | 72 | 22.2 | 10.8–33.7 | 113 | 9.7 | 2.4–17.0 | 100 | 11.2 | 3.0–19.5 | 56 | 4.9 | 0.0–11.4 | |||||||
| Taiwan* | 5,078 | 30.3 | 28.7–31.9 | 10,154 | 28.0 | 26.7–29.3 | 13,817 | 29.1 | 27.9–30.3 | 16,304 | 25.6 | 24.5–26.8 | 11,787 | 15.2 | 13.8–16.6 | ||||
| Thai registries | 83 | 12.6 | 4.2–21.0 | 72 | 14.2 | 3.0–25.4 | |||||||||||||
| Turkey (Izmir) | 80 | 24.4 | 11.9–37.0 | 105 | 31.4 | 19.7–43.1 | 69 | 12.7 | 1.9–23.4 | ||||||||||
| Europe | |||||||||||||||||||
| Austria* | 50 | 28.6 | 14.5–42.7 | 255 | 18.1 | 12.6–23.6 | 582 | 17.7 | 13.4–22.0 | 877 | 13.3 | 9.8–16.7 | 835 | 8.6 | 5.2–12.1 | ||||
| Belgium* | 86 | 31.7 | 18.2–45.2 | 207 | 39.5 | 30.3–48.7 | 565 | 30.1 | 22.7–37.6 | 727 | 17.9 | 12.5–23.3 | 578 | 13.4 | 7.6–19.1 | ||||
| Denmark* | 78 | 11.9 | 3.8–20.0 | 207 | 7.4 | 1.8–13.1 | 275 | 5.3 | 1.4–9.2 | 226 | 4.2 | 0.0–9.3 | |||||||
| Finland* | 210 | 10.5 | 3.1–17.9 | 340 | 15.7 | 10.1–21.4 | 388 | 7.3 | 2.3–12.4 | ||||||||||
| French registries | 94 | 25.8 | 15.0–36.7 | 275 | 27.4 | 21.0–33.7 | 400 | 18.3 | 13.6–23.0 | 306 | 14.4 | 8.9–19.8 | |||||||
| German registries | 61 | 39.9 | 25.6–54.1 | 266 | 22.4 | 15.4–29.4 | 608 | 19.9 | 14.8–25.1 | 1,165 | 13.5 | 10.4–16.7 | 992 | 8.0 | 4.2–11.8 | ||||
| Ireland* | 74 | 37.2 | 21.3–53.1 | 84 | 10.2 | 0.1–20.3 | 75 | 9.5 | 0.0–19.0 | ||||||||||
| Italian registries | 143 | 43.7 | 34.0–53.3 | 519 | 32.7 | 27.5–37.9 | 1,462 | 29.5 | 26.3–32.7 | 2,783 | 20.6 | 18.4–22.9 | 2,498 | 14.7 | 12.3–17.1 | ||||
| Netherlands* | 78 | 27.3 | 15.2–39.5 | 133 | 20.8 | 9.4–32.1 | 293 | 19.5 | 12.2–26.7 | 353 | 13.0 | 6.9–19.1 | 331 | 3.2 | 0.0–6.7 | ||||
| Norway* | 58 | 20.4 | 8.1–32.8 | 91 | 16.8 | 6.4–27.1 | 119 | 15.3 | 6.2–24.4 | 196 | 5.8 | 0.9–10.7 | |||||||
| Polish registries | 144 | 18.5 | 10.6–26.4 | 256 | 15.8 | 8.9–22.6 | 499 | 8.7 | 4.2–13.1 | 598 | 5.8 | 2.4–9.3 | 352 | 8.9 | 3.1–14.7 | ||||
| Portugal* | 62 | 25.7 | 13.5–37.8 | 183 | 25.3 | 17.3–33.3 | 317 | 19.1 | 13.6–24.5 | 454 | 11.6 | 6.8–16.4 | 273 | 14.4 | 7.2–21.6 | ||||
| Slovenia* | 50 | 20.8 | 6.8–34.8 | 93 | 0.3 | 0.0–0.8 | 130 | 12.2 | 3.3–21.1 | 75 | 0.2 | 0.0–0.8 | |||||||
| Spanish registries | 115 | 36.8 | 26.6–47.0 | 325 | 23.7 | 17.5–30.0 | 536 | 27.0 | 21.7–32.3 | 786 | 16.4 | 12.5–20.3 | 693 | 10.2 | 5.8–14.6 | ||||
| Sweden* | 141 | 32.0 | 23.2–40.8 | 330 | 20.7 | 14.6–26.8 | 457 | 11.0 | 6.7–15.3 | 577 | 7.4 | 4.1–10.7 | |||||||
| Swiss registries | 120 | 22.6 | 12.1–33.0 | 260 | 24.4 | 17.0–31.8 | 413 | 16.7 | 11.1–22.4 | 301 | 12.5 | 6.8–18.2 | |||||||
| United Kingdom* | 355 | 26.8 | 20.8–32.8 | 767 | 19.4 | 15.2–23.6 | 1,659 | 17.8 | 14.8–20.7 | 2,484 | 8.9 | 6.9–10.9 | 2,610 | 5.5 | 3.2–7.8 | ||||
| Oceania | |||||||||||||||||||
| Australian registries | 117 | 28.4 | 15.9–41.0 | 530 | 22.1 | 16.7–27.6 | 642 | 24.3 | 19.2–29.4 | 729 | 14.4 | 10.2–18.6 | 694 | 8.5 | 4.6–12.4 | ||||
| New Zealand* | 151 | 21.7 | 9.4–34.1 | 194 | 24.3 | 15.2–33.4 | 184 | 22.6 | 13.6–31.7 | 164 | 14.0 | 5.5–22.6 | |||||||
| Combined estimate††† | 16,290 | 30.6 | 28.3–33.0 | 47,747 | 24.6 | 22.4–26.9 | 65,341 | 21.4 | 18.3–24.5 | 66,727 | 15.8 | 13.7–17.8 | 49,020 | 10.2 | 8.4–12.1 | ||||
*, data with 100% coverage of the national population; †, microscopically verified (see text); ††, estimated with a random effects model (see text).
There is some evidence that age-standardised five-year survival tends to be slightly higher for women (21.8%) than men (17.5%; Table 5).
Table 5
| Region | Hepatocellular carcinoma | Cholangiocarcinoma | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | ||||||||||||
| No. | NS (%) | 95% CI | No. | NS (%) | 95% CI | No. | NS (%) | 95% CI | No. | NS (%) | 95% CI | ||||
| America (North) | |||||||||||||||
| Canada* | 3,411 | 24.2 | 21.8–26.6 | 918 | 28.3 | 23.8–32.7 | 632 | 5.6 | 2.9–8.2 | 543 | 10.0 | 6.5–13.5 | |||
| US registries | 43,715 | 17.1 | 16.4–17.7 | 13,041 | 20.7 | 19.6–21.9 | 5,056 | 8.2 | 7.0–9.4 | 4,607 | 9.8 | 8.3–11.2 | |||
| Asia | |||||||||||||||
| Chinese registries | 4,375 | 20.8 | 18.4–23.2 | 1,240 | 23.4 | 20.1–26.7 | 389 | 20.5 | 14.2–26.8 | 247 | 11.6 | 6.8–16.4 | |||
| Japanese registries | 9,864 | 25.1 | 23.8–26.5 | 4,357 | 28.2 | 26.1–30.2 | 876 | 11.4 | 8.7–14.2 | ||||||
| Korea* | 52,577 | 23.8 | 22.9–24.7 | 13,974 | 26.7 | 25.5–27.9 | 6,708 | 9.4 | 8.2–10.6 | 4,006 | 9.3 | 7.9–10.8 | |||
| Taiwan* | 41,436 | 23.4 | 22.6–24.1 | 15,704 | 27.3 | 26.2–28.5 | 2,440 | 8.6 | 6.9–10.3 | 2,155 | 8.2 | 6.4–9.9 | |||
| Europe | |||||||||||||||
| Austria* | 2,044 | 14.5 | 12.4–16.7 | 555 | 15.1 | 10.9–19.3 | 381 | 6.4 | 3.2–9.7 | ||||||
| Belgium* | 1,604 | 21.9 | 18.3–25.5 | 559 | 26.1 | 20.4–31.9 | 330 | 12.3 | 7.7–16.9 | ||||||
| Denmark* | 639 | 7.5 | 4.4–10.7 | ||||||||||||
| French registries | 955 | 20.0 | 16.9–23.1 | ||||||||||||
| German registries | 2,334 | 15.2 | 12.8–17.6 | 758 | 15.8 | 11.7–19.8 | 483 | 12.9 | 8.7–17.2 | 419 | 9.1 | 5.2–13.0 | |||
| Italian registries | 5,430 | 24.0 | 22.3–25.8 | 1,975 | 24.1 | 21.4–26.9 | 493 | 7.5 | 4.8–10.2 | ||||||
| Netherlands* | 894 | 11.4 | 8.0–14.9 | 294 | 18.6 | 13.5–23.8 | |||||||||
| Norway* | 351 | 11.7 | 7.5–16.0 | ||||||||||||
| Polish registries | 1,194 | 6.8 | 4.4–9.2 | 655 | 14.6 | 10.5–18.7 | 444 | 7.9 | 4.2–11.5 | 512 | 9.0 | 6.2–11.8 | |||
| Portugal* | 1,081 | 16.6 | 13.3–20.0 | ||||||||||||
| Spanish registries | 1,974 | 18.4 | 15.8–21.0 | ||||||||||||
| Sweden* | 1,112 | 16.5 | 13.8–19.2 | 412 | 5.1 | 2.8–7.5 | 404 | 6.1 | 3.2–9.1 | ||||||
| Swiss registries | 901 | 17.9 | 14.4–21.4 | ||||||||||||
| United Kingdom* | 6,176 | 12.0 | 10.5–13.5 | 1,699 | 14.3 | 11.8–16.8 | 3,170 | 4.9 | 3.6–6.2 | 3,530 | 5.7 | 4.3–7.1 | |||
| Oceania | |||||||||||||||
| Australian registries | 2,172 | 15.9 | 13.4–18.5 | 540 | 20.5 | 16.0–24.9 | 622 | 8.3 | 5.2–11.4 | 528 | 6.5 | 3.6–9.3 | |||
| New Zealand* | 595 | 18.4 | 13.3–23.5 | ||||||||||||
| Combined estimate† | 184,834 | 17.5 | 15.3–19.7 | 56,269 | 21.8 | 19.0–24.6 | 22,436 | 8.8 | 6.9–10.7 | 16,951 | 8.3 | 7.2–9.5 | |||
*, data with 100% coverage of the national population; †, estimated with a random effects model (see text).
In every country except Poland, five-year net survival for younger patients (15–44 years) diagnosed during 2004–2009 was higher than the pooled estimate for patients diagnosed in that age group some 10 years earlier, 1995–2000 (20.2%; Figure 5A). In Korea, Taiwan and Italy, this increase was seen in every age group (Figure 5A,B,C,D,E).
Cholangiocarcinoma
Age-standardised five-year net survival for patients diagnosed during 2004-2009 ranged from 3.7% in Thailand and Finland to 16.0% in China (Table 3; Figure 6A). The pooled estimate was 8.4%. Survival was similar for men (8.8%) and women (8.3%) (Table 5).

Five-year survival for patients diagnosed during 2004–2009 exceeded the upper 95% control limit for patients diagnosed during 1995–1999 in China (16.0%), Belgium (14.4%) and Portugal (13.3%) (pooled estimate 6.0%) (Figure 6B).
All the age-standardised five-year conditional survival estimates for 2004–2009 were within the control limits around the pooled estimate for patients diagnosed ten years earlier (22.0%), suggesting there had been little change in longer-term survival (Figure 6C).
Discussion
CONCORD-2 is the largest study to date of population-based survival from primary malignant neoplasms of the liver. The estimates of net survival up to five years after diagnosis presented here are based on data for 578,740 patients from 187 population-based registries in 36 countries over the 15-year period 1995–2009. All the estimates are corrected for international variation and trends in background mortality, and where possible they are age-standardised. For patients diagnosed during 2004–2009, age-standardised comparisons of net survival are now available for HCC in 25 countries and for cholangiocarcinoma in 20 countries.
The pooled estimate of age-standardised five-year net survival for primary liver cancer during 2004–2009 was 14.8% (range 4.4–23.7%). Survival was higher for patients diagnosed with HCC (17.4%, range 7.7–25.5%) than for those with cholangiocarcinoma (8.4%, range 3.7–16.0%).
Five-year net survival increased slightly between 1995–2000 (pooled estimate 11.0%) and 2004–2009 (14.8%), most noticeably in younger patients and for those with HCC. Given that survival is notably higher for HCC than for cholangiocarcinoma, and the wide international variation in the relative frequency of these two sub-types, international comparisons of liver cancer survival should probably be done separately for HCC and cholangiocarcinoma.
In Canada, Italy, Japan, Taiwan and Korea, five-year net survival for HCC (21.2–23.7%) in 2004–2009 was higher than the pooled estimate for 1995–2000. Japan introduced a programme for early diagnosis with new imaging techniques from the 1980s, with advanced techniques in surgery and chemotherapy (35). The proportion of tumours larger than 10 cm fell from 65.0% to 6.0% during 1978–2005 (36). The proportion of patients diagnosed with localised disease in Japan (60%) (35) is higher than in Korea (44%) (37), the USA (41%) (38) or Taiwan (30%) (39). The evidence of reduced mortality from screening patients with chronic liver disease is weak (40), but a dose-dependent association was found in a national study in Taiwan between shorter intervals from ultrasonography examination to a confirmed diagnosis and subsequent mortality (41). The high proportion of DCO registrations in Japan (20.5%) and the low proportion of patients with histological confirmation of the diagnosis in Italy (43.5%) may have modified the stage distribution (data not shown), but we have not examined survival by stage.
Age-standardised 5-year net survival for HCC was slightly but systematically higher for women than for men. A similar result was seen in the US SEER programme from a study of 39,345 patients diagnosed between 1988 and 2010, in which the hazard ratio for all-cause survival was 17% lower in women than men (42). The role of sex hormones was invoked in that study, but earlier detection could also play a role.
Conditional survival (five-year net survival among patients who had survived to the first anniversary of diagnosis) in 2004–2009 was highest in New Zealand, Canada, Taiwan, Korea, and China (42.0–52.7%). International variation in conditional five-year survival from HCC is likely to reflect the impact of variation in treatment for earlier-stage disease better than variation in five-year survival estimates that include the first year, because many patients with advanced-stage disease will have died in the first year after diagnosis. It may also reflect variation in treatment for localised and early-stage disease, through wider access to surgery (38,43,44), including liver transplantation (45,46), better patient selection (47-49) and clinical experience (50-52). Almost all these studies were done in Taiwan or the US.
Five-year net survival for patients diagnosed with cholangiocarcinoma during 2004–2009 was extremely low world-wide (3.7–16.0%). Survival in China, Belgium and Portugal has improved since 1995–2000, but little improvement has been seen in most other countries. Improvements in survival have been reported from the SEER programme in the USA (53), but most patients still receive no liver-directed intervention (54), despite evidence of better outcomes from resection (17) and transplantation (55). Resection rates have not improved (17), and barriers to treatment, such as household income (56) have been identified. Again, most of these are studies are from the US.
This study has highlighted the wide variation in data quality for liver cancer from population-based cancer registries. The problem arises partly because liver cancers are often diagnosed late, when invasive investigation is not warranted, survival is poor and the proportion of cases registered only from a death certificate (DCO) can be high. The liver is also a site of predilection for metastasis from other organs. These aspects of data quality can affect the comparability of survival estimates, both by exclusion of DCOs, for which the duration of survival is unknown but probably very short, and by the inability to determine accurately the morphologic type or the stage at diagnosis. Variability in data quality was also shown by the coding of cholangiocarcinoma to the liver parenchyma (20–30% of cases in four countries).
Misclassification of liver metastases as primary liver cancers will have been reduced by the exclusion of patients for whom the only basis of diagnosis was a death certificate. We also excluded patients for whom there was no evidence of microscopic verification. The European Network of Cancer Registries recommends assignation of a morphology code for HCC (ICD-O-3 M8170) if a liver tumour is diagnosed solely from high levels of alpha-fetoprotein, so some primary HCCs may have been excluded where this practice was not adopted. Survival estimates are more susceptible to bias when a large proportion of patients is excluded, such as in Romania, Thailand, Japan, Italy and China. Incomplete trace-back to find the date of diagnosis of cases first notified to the registry from a death certificate, resulting in a high proportion of DCO registrations, has been shown to bias survival estimates upwards, because such cases are often diagnosed shortly before death, leaving little time for routine cancer registration (57,58). By contrast, Denmark undertakes very intensive trace-back; the proportion of DCO cases for liver cancer is extremely low (0.8%), and this leads to inclusion in the analyses of many patients with very short survival.
Funnel plots are preferable to ranked bar charts for displaying survival estimates as higher or lower than a given benchmark, because they take due account of the precision of each estimate (34). Here, we devised a new method, using a random effects model to handle the wide international variation in both the survival estimates and the precision of those estimates, while maintaining control limits within the range 0 to 100%. The more objective comparison of survival estimates, presented alongside information on data quality, should motivate adoption of better registration practice, to improve both completeness and quality of the data. The collection of more complete data on tumour stage needs special emphasis, to enable evaluation of the contributions of early diagnosis and timely treatment to survival (59,60).
Unfortunately, many countries in Asia and Africa, where liver cancer incidence is usually high, could not be included in the analyses because of the lack of population-based cancer registry data. Survival in these countries is likely to be lower than in the high-income countries from which most of the data presented here were available (61).
Conclusions
Despite international variation and improvement over time, survival from liver cancer remains very low in most countries, particularly for cholangiocarcinoma. For hepatocellular carcinoma, prevention remains an urgent priority, through reduction in exposure to key risk factors such as aflatoxin (62), responsible for 5–28% of cases (4), and excessive alcohol consumption (63,64), as well as more widespread immunization against hepatitis B and C (14). Difficulties in implementing vaccination in low- and middle-income countries suggest that the incidence of hepatocellular carcinoma is likely to remain high (13).
Improving survival should therefore remain a high priority. Credible international comparisons of survival should stimulate policy to improve early diagnosis, and clinical trials of new approaches to treatment. Sustained effort is required to expand population-based cancer registration for surveillance of cancer incidence and survival worldwide. Global studies of cancer survival, such as CONCORD, contribute to this effort.
Table S1
| Region | Total§ No. | Morphology† | Topography†† | Liver | Intrahepatic bile ducts§ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocellular§ | Cholangiocarcinoma§ | Liver | Intrahepatic bile ducts§ | HCC | CC | HCC | CC | |||||||||||
| No. | % | No. | % | No. | % | No. | % | % | % | % | % | |||||||
| America (Central and South) | ||||||||||||||||||
| Colombia (Cali) | 458 | 241 | 52.6 | 156 | 34.1 | 319 | 69.7 | 139 | 30.3 | 75.5 | 9.7 | 0.0 | 89.9 | |||||
| America (North) | ||||||||||||||||||
| Canada* | 11,902 | 8,777 | 73.7 | 2,277 | 19.1 | 9,919 | 83.3 | 1,983 | 16.7 | 88.5 | 4.6 | 0.0 | 91.6 | |||||
| US registries | 140,046 | 106,667 | 76.2 | 21,066 | 15.0 | 123,488 | 88.2 | 16,558 | 11.8 | 86.4 | 5.2 | 0.0 | 88.4 | |||||
| Asia | ||||||||||||||||||
| Chinese registries | 10,569 | 6,870 | 65.0 | 800 | 7.6 | 9,920 | 93.9 | 649 | 6.1 | 69.1 | 2.7 | 2.0 | 81.7 | |||||
| Indonesia (Jakarta) | 85 | 63 | 74.1 | <5 | 4.7 | 83 | 97.6 | <5 | 2.4 | 75.9 | 2.4 | 0.0 | 100.0 | |||||
| Japanese registries | 19,882 | 17,483 | 87.9 | 1,648 | 8.3 | 18,228 | 91.7 | 1,654 | 8.3 | 95.9 | 0.8 | 0.4 | 90.7 | |||||
| Korea* | 160,125 | 134,561 | 84.0 | 18,036 | 11.3 | 138,431 | 86.5 | 21,694 | 13.5 | 97.2 | 1.0 | 0.0 | 76.9 | |||||
| Malaysia (Penang) | 814 | 615 | 75.6 | 110 | 13.5 | 728 | 89.4 | 86 | 10.6 | 82.8 | 6.5 | 14.0 | 73.3 | |||||
| Mongolia* | 422 | 119 | 28.2 | <5 | 0.9 | 421 | 99.8 | <5 | 0.2 | 28.3 | 0.7 | 0.0 | 100.0 | |||||
| Taiwan* | 99,383 | 89,109 | 89.7 | 7,941 | 8.0 | 91,659 | 92.2 | 7,724 | 7.8 | 97.2 | 0.8 | 0.0 | 93.0 | |||||
| Thai registries | 1,614 | 406 | 25.2 | 1,088 | 67.4 | 674 | 41.8 | 940 | 58.2 | 60.2 | 30.4 | 0.0 | 93.9 | |||||
| Turkey (Izmir) | 736 | 588 | 79.9 | 90 | 12.2 | 625 | 84.9 | 111 | 15.1 | 94.1 | 0.2 | 0.0 | 80.2 | |||||
| Europe | ||||||||||||||||||
| Austria* | 9,184 | 6,162 | 67.1 | 1,656 | 18.0 | 7,309 | 79.6 | 1,875 | 20.4 | 84.3 | 3.8 | 0.0 | 73.5 | |||||
| Belgium* | 2,958 | 2,163 | 73.1 | 607 | 20.5 | 2,341 | 79.1 | 617 | 20.9 | 92.4 | 1.2 | 0.0 | 94.0 | |||||
| Denmark* | 3,519 | 1,838 | 52.2 | 945 | 26.9 | 3,157 | 89.7 | 362 | 10.3 | 58.1 | 20.7 | 1.4 | 80.1 | |||||
| Estonia* | 609 | 277 | 45.5 | 154 | 25.3 | 433 | 71.1 | 176 | 28.9 | 64.0 | 3.9 | 0.0 | 77.8 | |||||
| Finland* | 3,434 | 2,086 | 60.7 | 818 | 23.8 | 2,720 | 79.2 | 714 | 20.8 | 76.7 | 6.3 | 0.0 | 90.8 | |||||
| French registries | 6,500 | 5,337 | 82.1 | 847 | 13.0 | 5,777 | 88.9 | 723 | 11.1 | 92.4 | 2.4 | 0.0 | 97.6 | |||||
| German registries | 7,034 | 4,996 | 71.0 | 1,241 | 17.6 | 5,580 | 79.3 | 1,454 | 20.7 | 89.5 | 1.8 | 0.0 | 78.3 | |||||
| Ireland* | 811 | 524 | 64.6 | 226 | 27.9 | 568 | 70.0 | 243 | 30.0 | 92.3 | 1.8 | 0.0 | 88.9 | |||||
| Italian registries | 24,401 | 20,100 | 82.4 | 2,069 | 8.5 | 22,851 | 93.6 | 1,550 | 6.4 | 88.0 | 3.1 | 0.0 | 87.4 | |||||
| Malta* | 66 | 24 | 36.4 | 19 | 28.8 | 45 | 68.2 | 21 | 31.8 | 53.3 | 4.4 | 0.0 | 81.0 | |||||
| Netherlands* | 3,557 | 2,674 | 75.2 | 647 | 18.2 | 2,974 | 83.6 | 583 | 16.4 | 89.9 | 2.6 | 0.0 | 97.8 | |||||
| Norway* | 1,583 | 1,099 | 69.4 | 376 | 23.8 | 1,194 | 75.4 | 389 | 24.6 | 92.0 | 0.0 | 0.0 | 96.7 | |||||
| Polish registries | 5,553 | 2,749 | 49.5 | 1,607 | 28.9 | 4,926 | 88.7 | 627 | 11.3 | 55.6 | 22.0 | 1.3 | 83.1 | |||||
| Portugal* | 3,285 | 2,337 | 71.1 | 580 | 17.7 | 2,827 | 86.1 | 458 | 13.9 | 82.7 | 7.2 | 0.0 | 82.1 | |||||
| Romania (Cluj) | 142 | 106 | 74.6 | 28 | 19.7 | 112 | 78.9 | 30 | 21.1 | 94.6 | 2.7 | 0.0 | 83.3 | |||||
| Russia (Arkhangelsk) | 119 | 55 | 46.2 | 29 | 24.4 | 98 | 82.4 | 21 | 17.6 | 56.1 | 11.2 | 0.0 | 85.7 | |||||
| Slovakia* | 125 | 81 | 64.8 | 38 | 30.4 | 86 | 68.8 | 39 | 31.2 | 94.2 | 2.3 | 0.0 | 92.3 | |||||
| Slovenia* | 1,086 | 758 | 69.8 | 218 | 20.1 | 877 | 80.8 | 209 | 19.2 | 86.4 | 5.7 | 0.0 | 80.4 | |||||
| Spanish registries | 7,811 | 6,250 | 80.0 | 987 | 12.6 | 6,813 | 87.2 | 998 | 12.8 | 91.7 | 2.5 | 0.0 | 82.1 | |||||
| Sweden* | 7,044 | 4,005 | 56.9 | 2,245 | 31.9 | 7,044 | 100.0 | 0 | 0.0 | 56.9 | 31.9 | |||||||
| Swiss registries | 3,095 | 2,536 | 81.9 | 403 | 13.0 | 2,717 | 87.8 | 378 | 12.2 | 93.3 | 2.2 | 0.0 | 91.0 | |||||
| United Kingdom* | 29,912 | 15,159 | 50.7 | 13,143 | 43.9 | 16,180 | 54.1 | 13,732 | 45.9 | 93.7 | 1.0 | <0.1 | 94.5 | |||||
| Oceania | ||||||||||||||||||
| Australian registries | 8,845 | 6,316 | 71.4 | 2,145 | 24.3 | 6,625 | 74.9 | 2,220 | 25.1 | 95.3 | 0.6 | 0.0 | 94.8 | |||||
| New Zealand* | 2,031 | 1,403 | 69.1 | 576 | 28.4 | 1,443 | 71.0 | 588 | 29.0 | 97.2 | 0.7 | 0.0 | 96.3 | |||||
§, microscopically confirmed (see text); *, data with 100% coverage of the national population; †, hepatocellular carcinoma: ICD-O-3 morphology codes 8170–8175; cholangiocarcinoma: 8050, 8140–8141, 8160–8161, 8260, 8440, 8480–8500 and 8570–8572; ††, liver: ICD-O-3 topography code C22.0; intrahepatic bile ducts C22.1.
Acknowledgments
We thank Lisa Montel and Adrian Turculeț for logistical support.
Funding: Canadian Partnership Against Cancer (Toronto, Canada), Cancer Focus Northern Ireland (Belfast, UK), Cancer Institute New South Wales (Sydney, Australia), Cancer Research UK (London, UK), Centers for Disease Control and Prevention (Atlanta GA, USA), Swiss Re (London, UK), Swiss Cancer Research foundation (Bern, Switzerland), Swiss Cancer League (Bern, Switzerland), and University of Kentucky (Lexington KY, USA).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Wanqing Chen) for the series “Global Cancer Burden” published in Annals of Cancer Epidemiology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace.2019.07.01). The series “Global Cancer Burden” was commissioned by the editorial office without any funding or sponsorship. M. Coleman serves as an unpaid editorial board member of Annals of Cancer Epidemiology. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Confidentiality Advisory Group of the UK’s Statutory Health Research Authority (HRA; reference ECC 3-04(i)/2011; last update March 3, 2017), the National Health Service Research Ethics Service (11/LO/0331; Feb 21, 2017), and the London School of Hygiene & Tropical Medicine Ethics Committee (12171; Sept 6, 2017).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [PubMed]
- Katanoda K, Hori M. Morphological distribution of liver cancer from Cancer Incidence in Five Continents Vol. X. Jpn J Clin Oncol 2015;45:607. [Crossref] [PubMed]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-73.e1. [Crossref] [PubMed]
- Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect 2010;118:818-24. [Crossref] [PubMed]
- Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323-31. [Crossref] [PubMed]
- Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 2012;130:1639-48. [Crossref] [PubMed]
- Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol 2008;24:349-56. [Crossref] [PubMed]
- Wadsworth CA, Lim A, Taylor-Robinson SD, et al. The risk factors and diagnosis of cholangiocarcinoma. Hepatol Int 2013;7:377-93. [Crossref] [PubMed]
- Cai H, Kong WT, Chen CB, et al. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: a meta-analysis of observational studies. BMC Cancer 2015;15:831. [Crossref] [PubMed]
- Li H, Hu B, Zhou ZQ, et al. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol 2015;13:161. [Crossref] [PubMed]
- Ye XH, Huai JP, Ding J, et al. Smoking, alcohol consumption, and the risk of extrahepatic cholangiocarcinoma: a meta-analysis. World J Gastroenterol 2013;19:8780-8. [Crossref] [PubMed]
- Parsi MA. Obesity and cholangiocarcinoma. World J Gastroenterol 2013;19:457-62. [Crossref] [PubMed]
- Lahariya C, Subramanya BP, Sosler S. An assessment of hepatitis B vaccine introduction in India: lessons for roll out and scale up of new vaccines in immunization programs. Indian J Public Health 2013;57:8-14. [Crossref] [PubMed]
- World Health Organisation. Hepatitis B vaccines: WHO position paper, July 2017 - Recommendations. Vaccine 2019;37:223-5. [Crossref] [PubMed]
- Bah E, Carrieri MP, Hainaut P, et al. 20-years of population-based cancer registration in hepatitis B and liver cancer prevention in the Gambia, West Africa. PLoS One 2013;8:e75775 [Crossref] [PubMed]
- Coleman MP. Cancer survival: global surveillance will stimulate health policy and improve equity. Lancet 2014;383:564-73. [Crossref] [PubMed]
- Anderson JE, Hemming AW, Chang DC, et al. Surgical management trends for cholangiocarcinoma in the USA 1998-2009. J Gastrointest Surg 2012;16:2225-32. [Crossref] [PubMed]
- Lepage C, Capocaccia R, Hackl M, et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: Results of EUROCARE-5. Eur J Cancer 2015;51:2169-78. [Crossref] [PubMed]
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Fritz AG, Percy C, Jack A, et al. editors. International Classification of Diseases for Oncology (ICD-O). 3rd ed. Geneva: World Health Organization;, 2000.
- Tyczynski JE, Démaret E, Parkin DM. Standards and guidelines for cancer registration in Europe. The ENCR recommendations. IARC Technical Publication No. 40. Lyon: IARC, 2003.
- Forman D, Bray F, Brewster DH, et al. editors. Cancer incidence in five continents. Vol. X. (IARC Scientific Publications No. 164). Lyon: International Agency for Research on Cancer, 2014.
- Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics 2012;68:113-20. [Crossref] [PubMed]
- StataCorp. STATA statistical software. 14 ed. College Station TX: Stata Corporation, 2015.
- Clerc-Urmès I, Grzebyk M, Hédelin G. Net survival estimation with stns. Stata J 2014;14:87-102. [Crossref]
- Spika D, Rachet B, Bannon F, et al. Life tables for the CONCORD-2 study. London: CONCORD Central Analytic Team, 2015. Available online: http://csg.lshtm.ac.uk/tools-analysis/ (accessed 10 May 2018).
- Brenner H, Gefeller O. Deriving more up-to-date estimates of long-term patient survival. J Clin Epidemiol 1997;50:211-6. [Crossref] [PubMed]
- Allemani C, Harewood R, Johnson C, et al. Population-based cancer survival in the US: data, quality control and statistical methods. Cancer 2017;123:4982-93. [Crossref] [PubMed]
- Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307-16. [Crossref] [PubMed]
- Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med 2005;24:1185-202. [Crossref] [PubMed]
- Spiegelhalter DJ. Handling over-dispersion of performance indicators. Qual Saf Health Care 2005;14:347-51. [Crossref] [PubMed]
- Quaresma M, Coleman MP, Rachet B. Funnel plots for population-based cancer survival: principles, methods and applications. Stat Med 2014;33:1070-80. [Crossref] [PubMed]
- Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer 2015;4:39-50. [Crossref] [PubMed]
- Kudo M, Izumi N, Sakamoto M, et al. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer 2016;5:190-7. [Crossref] [PubMed]
- Jung KW, Won YJ, Kong HJ, et al. Survival of Korean adult cancer patients by stage at diagnosis, 2006-2010: national cancer registry study. Cancer Res Treat 2013;45:162-71. [Crossref] [PubMed]
- Altekruse SF, McGlynn KA, Dickie LA, et al. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology 2012;55:476-82. [Crossref] [PubMed]
- Chiang CJ, Lo WC, Yang YW, et al. Incidence and survival of adult cancer patients in Taiwan, 2002-2012. J Formos Med Assoc 2016;115:1076-88. [Crossref] [PubMed]
- Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med 2014;161:261-9. [Crossref] [PubMed]
- Wu CY, Hsu YC, Ho HJ, et al. Association between ultrasonography screening and mortality in patients with hepatocellular carcinoma: a nationwide cohort study. Gut 2016;65:693-701. [Crossref] [PubMed]
- Yang D, Hanna DL, Usher J, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer 2014;120:3707-16. [Crossref] [PubMed]
- Chiu CC, Wang JJ, Chen YS, et al. Trends and predictors of outcomes after surgery for hepatocellular carcinoma: A nationwide population-based study in Taiwan. Eur J Surg Oncol 2015;41:1170-8. [Crossref] [PubMed]
- Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. Journal of the National Medical Association 2006;98:1934-9. [PubMed]
- Devaki P, Wong RJ, Marupakula V, et al. Approximately one-half of patients with early-stage hepatocellular carcinoma meeting Milan criteria did not receive local tumor destructive or curative surgery in the post-MELD exception era. Cancer 2014;120:1725-32. [Crossref] [PubMed]
- Wong RJ, Devaki P, Nguyen L, et al. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl 2014;20:528-35. [Crossref] [PubMed]
- Wang JH, Changchien CS, Hu TH, et al. The efficacy of treatment schedules according to Barcelona Clinic Liver Cancer staging for hepatocellular carcinoma - Survival analysis of 3892 patients. Eur J Cancer 2008;44:1000-6. [Crossref] [PubMed]
- Yi PS, Zhang M, Zhao JT, et al. Liver resection for intermediate hepatocellular carcinoma. World J Hepatol 2016;8:607-15. [Crossref] [PubMed]
- Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 2015;62:617-24. [Crossref] [PubMed]
- Sato M, Tateishi R, Yasunaga H, et al. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: a national survey of 54,145 patients. J Gastroenterol 2012;47:1125-33. [Crossref] [PubMed]
- Lu CC, Chiu CC, Wang JJ, et al. Volume-outcome associations after major hepatectomy for hepatocellular carcinoma: a nationwide Taiwan study. J Gastrointest Surg 2014;18:1138-45. [Crossref] [PubMed]
- Lu LC, Shao YY, Kuo RN, et al. Hospital volume of percutaneous radiofrequency ablation is closely associated with treatment outcomes for patients with hepatocellular carcinoma. Cancer 2013;119:1210-6. [Crossref] [PubMed]
- Amini N, Ejaz A, Spolverato G, et al. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol 2014;110:163-70. [Crossref] [PubMed]
- Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol 2008;15:600-8. [Crossref] [PubMed]
- Salgia RJ, Singal AG, Fu S, et al. Improved post-transplant survival in the United States for patients with cholangiocarcinoma after 2000. Dig Dis Sci 2014;59:1048-54. [Crossref] [PubMed]
- Kneuertz PJ, Kao LS, Ko TC, et al. Regional disparities affect treatment and survival of patients with intrahepatic cholangiocarcinoma--a Texas Cancer Registry analysis. J Surg Oncol 2014;110:416-21. [Crossref] [PubMed]
- Berrino F, Estève J, Coleman MP. Basic issues in the estimation and comparison of cancer patient survival. In: Berrino F, Sant M, Verdecchia A, et al. editors. Survival of cancer patients in Europe: the EUROCARE study (IARC Scientific Publications No 132). Lyon: International Agency for Research on Cancer (WHO), 1995:1-14.
- Robinson D, Sankila R, Hakulinen T, et al. Interpreting international comparisons of cancer survival: the effects of incomplete registration and the presence of death certificate only cases on survival estimates. Eur J Cancer 2007;43:909-13. [Crossref] [PubMed]
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the United Kingdom: a population-based study, 2004-2007. Thorax 2013;68:551-64. [Crossref] [PubMed]
- Maringe C, Walters S, Rachet B, et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-7. Acta Oncol 2013;52:919-32. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- International Agency for Research and Cancer. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 82). Lyon, France: IARC, 2002:171-300.
- London WT, McGlynn KA. Liver cancer. In: Schottenfeld D, Fraumeni JF. editors. Cancer Epidemiology and Prevention. Oxford: Oxford University Press, 2006:763-86.
- Anon. European Code Against Cancer. Lyon: International Agency for Research on Cancer, 2014.
Cite this article as: Bannon F, Di Carlo V, Harewood R, Engholm G, Ferretti S, Johnson CJ, Aitken JF, Marcos-Gragera R, Bonaventure A, Gavin A, Huws D, Coleman MP, Allemani C; CONCORD Working Group. Survival trends for primary liver cancer, 1995–2009: analysis of individual data for 578,740 patients from 187 population-based registries in 36 countries (CONCORD-2). Ann Cancer Epidemiol 2019;3:6.


